Immune Modulation Related to High-Dose Valacyclovir Administration for Primary Cytomegalovirus Infection in Pregnancy: An Insight into Virus Behavior and Maternal Serology
- PMID: 40006912
- PMCID: PMC11861677
- DOI: 10.3390/v17020157
Immune Modulation Related to High-Dose Valacyclovir Administration for Primary Cytomegalovirus Infection in Pregnancy: An Insight into Virus Behavior and Maternal Serology
Abstract
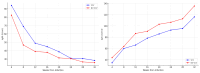
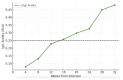
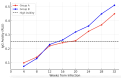
Cytomegalovirus (CMV) infection during pregnancy poses significant maternal and fetal health risks. Valacyclovir, an antiviral drug, has been explored as a therapeutic option for managing primary CMV infections in pregnant women. This study investigates the effects of valacyclovir therapy on immune response maturation against CMV, maternal antibody levels, and viral replication during treatment. We conducted a retrospective observational study involving pregnant women diagnosed with primary CMV infection and presenting in utero infection who received high-dose valacyclovir therapy (8 g/day). A group started the therapy at diagnosis, while another group started only after positive amniocentesis. Maternal antibody levels (IgM, IgG, and IgG avidity) and PCR for CMV testing (in blood, urine, and saliva) were measured longitudinally during the second and third trimesters. Our findings indicate that early valacyclovir therapy is related to lower avidity levels over time and a delay in reaching a high IgG avidity level (18.22 ± 1.21 weeks) compared to the patients who started Valacyclovir during the second trimester after positive amniocentesis (14.52 ± 1.64 weeks; p = 0.066). The therapy does not condition the overall concentration of maternal CMV-specific IgM and IgG. While high-dose VCV does not directly target the mechanism of IgG avidity maturation, it can interfere with this process by reducing the viral load and antigen presentation, influencing IgG avidity maturation. Further research is needed to elucidate the long-term implications of potential immunological modulation induced by Valacyclovir and to optimize early diagnosis and the right treatment protocol during pregnancy.
Keywords: IgG avidity; cytomegalovirus; immune system; pregnancy; valacyclovir.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Reliability of CMV-IgG kinetics in the diagnosis of CMV primary infection: sensitivity, specificity, and clinical implications.Microbiol Spectr. 2025 Aug 5;13(8):e0045525. doi: 10.1128/spectrum.00455-25. Epub 2025 Jun 17. Microbiol Spectr. 2025. PMID: 40525841 Free PMC article.
-
First-Trimester Universal One-Time Serology Screening for Cytomegalovirus: A Pilot Study at Two Tertiary Referral Centers in Barcelona (Catalunya, Spain).Fetal Diagn Ther. 2025;52(4):388-396. doi: 10.1159/000544169. Epub 2025 Feb 18. Fetal Diagn Ther. 2025. PMID: 39965551 Free PMC article.
-
Effectiveness and safety of prenatal valacyclovir for congenital cytomegalovirus infection: systematic review and meta-analysis.Ultrasound Obstet Gynecol. 2023 Apr;61(4):436-444. doi: 10.1002/uog.26136. Ultrasound Obstet Gynecol. 2023. PMID: 36484439
-
Provider-Led Interventions to Reduce Congenital Cytomegalovirus.J Midwifery Womens Health. 2025 Jul-Aug;70(4):576-592. doi: 10.1111/jmwh.13749. Epub 2025 Mar 28. J Midwifery Womens Health. 2025. PMID: 40152197 Free PMC article. Review.
-
[Secondary Prevention of Fetal Cytomegalovirus Infection Through Valacyclovir Administration in Maternal Primary Infections During the Periconceptional Period and First Trimester of Pregnancy].Gynecol Obstet Fertil Senol. 2025 Jul-Aug;53(7-8):341-348. doi: 10.1016/j.gofs.2025.03.010. Epub 2025 Apr 3. Gynecol Obstet Fertil Senol. 2025. PMID: 40185476 Review. French.
References
-
- Ssentongo P., Hehnly C., Birungi P., Roach M.A., Spady J., Fronterre C., Wang M., Murray-Kolb L.E., Al-Shaar L., Chinchilli V.M., et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries With Universal Screening A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021;4:e2120736. doi: 10.1001/jamanetworkopen.2021.20736. - DOI - PMC - PubMed
-
- Istituto Superiore di Sanità . La Gravidanza Fisiologica, Parte I. Antenatal Care for Uncomplicated Pregnancy. Sistema Nazionale Linee Guida (SNLG); Rome, Italy: 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

