The Combination of GS-441524 (Remdesivir) and Ribavirin Results in a Potent Antiviral Effect Against Human Parainfluenza Virus 3 Infection in Human Airway Epithelial Cell Cultures and in a Mouse Infection Model
- PMID: 40006927
- PMCID: PMC11860817
- DOI: 10.3390/v17020172
The Combination of GS-441524 (Remdesivir) and Ribavirin Results in a Potent Antiviral Effect Against Human Parainfluenza Virus 3 Infection in Human Airway Epithelial Cell Cultures and in a Mouse Infection Model
Abstract
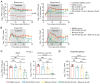
Human parainfluenza virus type 3 (HPIV-3) can cause severe respiratory diseases, particularly in young children, the elderly and immunocompromised. There are no approved antiviral drugs against this virus. We report that the combination of ribavirin with either remdesivir or its parent nucleoside GS-441524 results in a pronounced antiviral effect against HPIV-3 in LLC-MK2 cells and in human airway epithelial cells grown at the air-liquid interface. In AG129 mice intranasally inoculated with HPIV-3, the combined treatment with ribavirin and GS-441524 decreased infectious viral lung titers by >2.5 log10 to undetectable levels in 4 out of 11 mice and by 1.6 log10 in the remaining 7 mice as compared with the vehicle. The lungs of all mice that received the combined treatment appeared histologically normal or virtually normal, whereas 8 of 11 vehicle-treated mice presented with bronchopneumonia. By contrast, ribavirin alone did not result in a reduction in infectious viral lung titers; GS-441524 alone reduced infectious viral lung titers by 1.2 log10. Moreover, several mice in the single-treatment groups exhibited severe lung pathology. These findings may warrant exploring this combination in patients with severe HPIV-3 infections and possibly also against infections with other viruses that are susceptible in vitro to these two drugs.
Keywords: AG129 mice; human organoids; human respirovirus 3; obeldesivir; parainfluenza viruses; remdesivir; ribavirin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pan Y., Zhang Y., Shi W., Peng X., Cui S., Zhang D., Lu G., Liu Y., Wu S., Yang P., et al. Human parainfluenza virus infection in severe acute respiratory infection cases in Beijing, 2014–2016: A molecular epidemiological study. Influenza Other Respir. Viruses. 2017;11:564–568. doi: 10.1111/irv.12514. - DOI - PMC - PubMed
-
- Nichols W.G., Gooley T., Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: The Fred Hutchinson Cancer Research Center experience. Biol. Blood Marrow Transplant. 2001;7:11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

