Vaccinia Virus Vector Bivalent Norovirus Vaccine
- PMID: 40006992
- PMCID: PMC11861675
- DOI: 10.3390/v17020237
Vaccinia Virus Vector Bivalent Norovirus Vaccine
Abstract
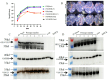
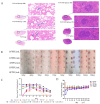
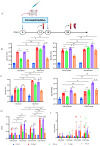
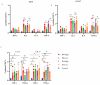
Norovirus is a major etiological agent of nonbacterial gastroenteritis around the world. Due to its in vitro culture complexity, high genome diversity, and the lack of cross-reactive immunity between genogroups, there is an unmet urgent need for polyvalent norovirus vaccines that provide broad-spectrum protection, and no vaccine has gained global approval to date. In this study, we constructed a bivalent norovirus vaccine, based on the highly attenuated poxvirus [strain VG9] vector, expressing the major capsid protein VP1 from genotypes GII.4 and GII.17. VG9-NOR exhibited a comparable replication ability to the authentic virus while preserving good safety. After the intramuscular and intranasal immunization of mice, VG9-NOR induced high IgG- and IgA-binding antibody (Ab) titers against GII.4 and GII.17, increased the secretion of GII.4 and GII.17-specific HGBA-blocking antibodies, and enhanced GII.17-specific mucosal immunity. Furthermore, VG9-NOR also induced a Th1-mediated cellular response. These results demonstrate that the polyvalent poxvirus vector vaccine expressing VP1 variants from different subtypes is able to elicit effective protection. Our study highlights the VG9 vector as a highly promising candidate for the development of polyvalent norovirus vaccines.
Keywords: bivalent vaccine; immune response; norovirus; poxvirus vector.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Pires S.M., Fischer-Walker C.L., Lanata C.F., Devleesschauwer B., Hall A.J., Kirk M.D., Duarte A.S.R., Black R.E., Angulo F.J. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE. 2015;10:e0142927. doi: 10.1371/journal.pone.0142927. - DOI - PMC - PubMed
-
- Chan M.C.W., Lee N., Hung T.-N., Kwok K., Cheung K., Tin E.K.Y., Lai R.W.M., Nelson E.A.S., Leung T.F., Chan P.K.S. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat. Commun. 2015;6:10061. doi: 10.1038/ncomms10061. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

