Adeno-Associated Virus Vectors: Principles, Practices, and Prospects in Gene Therapy
- PMID: 40006994
- PMCID: PMC11861813
- DOI: 10.3390/v17020239
Adeno-Associated Virus Vectors: Principles, Practices, and Prospects in Gene Therapy
Abstract
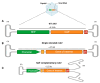
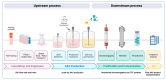
Gene therapy offers promising potential as an efficacious and long-lasting therapeutic option for genetic conditions, by correcting defective mutations using engineered vectors to deliver genetic material to host cells. Among these vectors, adeno-associated viruses (AAVs) stand out for their efficiency, versatility, and safety, making them one of the leading platforms in gene therapy. The enormous potential of AAVs has been demonstrated through their use in over 225 clinical trials and the FDA's approval of six AAV-based gene therapy products, positioning these vectors at the forefront of the field. This review highlights the evolution and current applications of AAVs in gene therapy, focusing on their clinical successes, ongoing developments, and the manufacturing processes required for the rapid commercial growth anticipated in the AAV therapy market. It also discusses the broader implications of these advancements for future therapeutic strategies targeting more complex and multi-systemic conditions and biological processes such as aging. Finally, we explore some of the major challenges currently confronting the field.
Keywords: adeno-associated virus (AAV); clinical trials; gene therapy; vector manufacturing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

