Serum-Free Suspension Culture of the Aedes albopictus C6/36 Cell Line for Chimeric Orthoflavivirus Vaccine Production
- PMID: 40007005
- PMCID: PMC11860912
- DOI: 10.3390/v17020250
Serum-Free Suspension Culture of the Aedes albopictus C6/36 Cell Line for Chimeric Orthoflavivirus Vaccine Production
Abstract
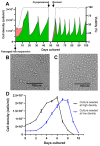
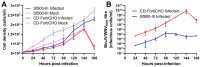
Chimeric orthoflaviviruses derived from the insect-specific Binjari virus (BinJV) offer a promising basis for safe orthoflavivirus vaccines. However, these vaccines have so far only been produced using adherent C6/36 Aedes albopictus mosquito cell cultures grown in serum-supplemented media, limiting their scalable manufacture. To address this, we adapted C6/36 cells for serum-free suspension culture using Sf900-III medium, achieving high peak cell densities (up to 2.5 × 107 cells/mL). Higher agitation rates reduced cell aggregation, and cryopreservation and direct-to-suspension revival were successful, confirming the adapted line's stability for research and industrial applications. Despite this, BinJV-based chimeric orthoflaviviruses, including BinJV/WNVKUN, a candidate vaccine for West Nile virus, and similar vaccines (BinJV/DENV2 and BinJV/JEVNSW22) for dengue 2 virus and Japanese encephalitis virus, respectively, exhibited substantially reduced titres in C6/36 cultures infected in Sf900-III, a phenomenon attributed to the medium's acidic pH. Switching to the more alkaline, serum-free CD-FortiCHO medium enhanced the replication of these chimeric viruses to peak titres between 1.7 × 107 and 7.6 × 109 infectious units per mL whilst preserving viral integrity. These findings suggest that suspension-adapted C6/36 cultures in CD-FortiCHO medium can support high-yield vaccine production for various orthoflaviviruses and highlight the important role of cell culture media pH for orthoflavivirus bioprocessing. This scalable mosquito cell-based system could reduce production costs and improve vaccine accessibility, supporting efforts to combat arbovirus-related public health challenges.
Keywords: C6/36; orthoflavivirus chimaeras; suspension culture; vaccines; virus bioprocessing.
Conflict of interest statement
The authors J.J.H., J.H.-P., and R.A.H. are inventors in a patent (WO/2018/176075) covering chimeric Binjari virus technology.
Figures

References
-
- Singh K.R.P. Cell cultures derived from larvae of Aedes albopictus (Skuse) and Aedes aegypti (L.) Curr. Sci. 1967;36:506–508.
-
- Brackney D.E., Scott J.C., Sagawa F., Woodward J.E., Miller N.A., Schilkey F.D., Mudge J., Wilusz J., Olson K.E., Blair C.D. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Neglected Trop. Dis. 2010;4:e856. doi: 10.1371/journal.pntd.0000856. - DOI - PMC - PubMed
-
- American Type Culture Collection . Aedes albopictus Clone C6/36 (ATCC CRL1660TM) ATCC; Manassas, VA, USA: 2017.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

