Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study
- PMID: 40007006
- PMCID: PMC11860205
- DOI: 10.3390/v17020251
Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study
Abstract
Introduction: Hepatitis D virus (HDV) infection remains a significant global health challenge due to its severity and high risk of progression to cirrhosis and hepatocellular carcinoma (HCC). Bulevirtide, a novel HDV entry inhibitor, has shown promise in managing chronic hepatitis D by blocking viral entry into hepatocytes. This study evaluated the efficacy and safety of bulevirtide in reducing HDV RNA levels and improving liver function in a real-life cohort of Italian patients with HDV infection.
Methods: This multicenter prospective trial enrolled 108 consecutive patients with chronic HDV infection, from June 2023 to June 2024, who received 2 mg/day of bulevirtide in combination with a nucleoside/nucleotide analogue for hepatitis B virus (HBV) infection. Patients with any stage of liver fibrosis or compensated cirrhosis were included. Data collected included demographic and clinical characteristics, liver function tests, HDV RNA levels, and adverse events at baseline and 6 months.
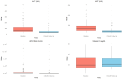
Results: The virological response was achieved in 54.6% of patients (n = 59), with 36 demonstrating undetectable HDV RNA levels. Among responders, ALT levels decreased significantly from 67.0 U/mL [IQR 44.0-116.3] to 31.5 U/mL [IQR 24.0-36.5, p = 0.001], and AST levels from 66.0 U/mL [IQR 46.5-91.0] to 32.5 U/mL [IQR 28.0-38.0, p = 0.021]. Median HDV RNA dropped from 29,800 IU/mL [IQR 3100-375,000] to 0 IU/mL [IQR 0-291, p < 0.001]. No significant predictors of response emerged. Mild adverse events, including pruritus (5.6%) and injection-site reactions (1.9%) and flu-like syndrome (0.9) were reported, with no treatment discontinuation.
Conclusions: Bulevirtide effectively reduces HDV RNA levels and improves liver function with a favorable safety profile, offering a promising therapeutic option for chronic hepatitis D. Further large-scale studies are needed to confirm these findings and explore long-term outcomes.
Keywords: antiviral treatment outcomes; bulevirtide therapy; hepatitis delta virus; liver disease management; safety and efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources