Endothelial damage and complement dysregulation in fetuses from pregnancies complicated by preeclampsia
- PMID: 40007223
- PMCID: PMC11981108
- DOI: 10.1111/aogs.15072
Endothelial damage and complement dysregulation in fetuses from pregnancies complicated by preeclampsia
Abstract
Introduction: Our objective was to evaluate the endothelial function profile and complement system in fetuses from preeclamptic pregnancies using ex vivo and in vitro approaches.
Material and methods: A total of 66 singleton pregnancies were prospectively recruited comprising 34 cases of preeclampsia and 32 normotensive pregnancies matched for baseline characteristics. In the ex vivo approach, soluble tumor necrosis factor-a receptor 1 (sTNFR1), vascular cell adhesion molecule-1 (sVCAM-1), intercellular adhesion molecule-1 (sICAM-1), Von Willebrand factor (sVWF), terminal complement complex (sC5b-9), Factor H, complement component C3a and Factor Bb were analyzed in fetal cord blood samples. In the in vitro model, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), Von Willebrand factor (VWF), vascular endothelial cadherin (VE-Cadherin), endothelial nitric oxide synthase (eNOS), reactive oxygen species (ROS) and C5b-9 deposits were evaluated on endothelial cells in culture exposed to fetal sera or plasma.
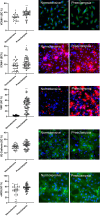
Results: Increased sVCAM-1, sICAM- l and decreased Factor H and Factor Bb concentrations were detected in preeclampsia fetuses as compared to fetuses from normotensive mothers (509.4 ± 28 vs. 378.4 ± 34.3 ng/mL, 161.1 ± 11.9 vs. 114.8 ± 6.8, 199.6 ± 18.3 vs. 267.1 ± 15.4 ng/mL and 6.6 ± 0.7 vs. 10.3 ± 1.4 μg/mL respectively, p < 0.05) with similar results in sTNFR1, sVWF, sC5b-9 and C3a. Endothelial cells exposed to fetal sera from preeclampsia showed incremented expression of VCAM-1(38.1 ± 1.4% vs. 28.3 ± 1.6%, p < 0.01), ICAM-1 (12 ± 0.9% vs. 8.6 ± 0.6%, p < 0.05), VWF (43.5 ± 2.9% vs. 3.7 ± 0.3%, p < 0.05), and ROS (5 × 1013 ± 1 × 1012 vs. 3.5 × 1013 ± 1.4 × 1012, p < 0.01) with similar expression of VE-Cadherin and eNOS as compared to those exposed to control fetuses. While soluble C5b-9 was similar between the study groups (851.4 ± 177.5 vs. 751.4 ± 132.81 ng/mL, p > 0.05), significantly less C5b-9 deposits on endothelial cells were induced by fetal plasma from preeclamptic compared to normotensive mothers (fold change 0.08 ± 0.02 vs. 0.48 ± 0.13, p < 0.01).
Conclusions: High levels of endothelial adhesion molecules and oxidative stress products suggest endothelial damage and reduced in vitro deposition of C5b-9 indicates complement dysregulation in preeclampsia fetuses. More research is necessary to study the impact of preeclampsia on fetal vascular health and innate immunity.
Keywords: C5b‐9; complement system; endothelium; fetus; preeclampsia; vascular.
© 2025 The Author(s). Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre‐eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. - PubMed
-
- Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med. 2022;386:1817‐1832. - PubMed
-
- Steegers EAP, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet. 2010;376:631‐644. - PubMed
MeSH terms
Substances
Grants and funding
- 202026-10/Fundació La Marató de TV3
- PI14/00226/Instituto de Salud Carlos III (ISCIII)
- PI17/00675/Instituto de Salud Carlos III (ISCIII)
- PI18/00073/Instituto de Salud Carlos III (ISCIII)
- PI20/00246/Instituto de Salud Carlos III (ISCIII)
- PI22/00684/Instituto de Salud Carlos III (ISCIII)
- PI22/00240/Instituto de Salud Carlos III (ISCIII)
- PI22/00109/Instituto de Salud Carlos III (ISCIII)
- INT21/00027/Instituto de Salud Carlos III (ISCIII)
- ERPR04G719/2016/Center of biomedical research on rare diseases
- Cerebra Foundation for the Brain Injured Child
- AP180722022/Fundación Mutua Madrileña
- 2021-SGR-01422/Departament d'Innovació, Universitats i Empresa, Generalitat de Catalunya
- FJC2021-048123-I/Ministry of Science, Innovation and Universities in Spain
- Fundación Jesus Serra del grupo Catalana Occident
LinkOut - more resources
Full Text Sources
Miscellaneous

