Sustained Interferon Signature Suppression With Anifrolumab in a Patient With STING-Associated Vasculopathy with Onset in Infancy Refractory to JAK Inhibitor and Dazukibart Therapy
- PMID: 40007227
- PMCID: PMC12311253
- DOI: 10.1002/art.43145
Sustained Interferon Signature Suppression With Anifrolumab in a Patient With STING-Associated Vasculopathy with Onset in Infancy Refractory to JAK Inhibitor and Dazukibart Therapy
Abstract
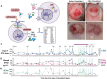
Objective: The objective was to report the safety and efficacy of an anti-IFNAR1 antibody (anifrolumab) in a patient with STING-associated vasculopathy with onset in infancy (SAVI) who presented with vasculitic ulcers and systemic inflammation refractory to JAK inhibition (JAKi) and to the interferon-β-neutralizing monoclonal antibody dazukibart.
Methods: A patient with SAVI and a de novo STING1 p.(Asn154Ser) mutation, a known pathogenic variant, and uncontrolled disease received 21 doses of dazukibart under a compassionate use investigational new drug protocol, which was followed by treatment with the anti-IFNAR1 antibody anifrolumab. Clinical and laboratory parameters, including wound healing, whole-blood type I interferon (IFN I) signature, and safety markers were closely monitored throughout both treatment periods.
Results: Despite initial reductions in C-reactive protein levels and IFN I scores following dazukibart administration, the patient experienced rebound inflammation and recurrent vasculitic lesions. Dazukibart dose adjustments failed to sustainably control IFN I signaling. Subsequent combination therapy of baricitinib and tocilizumab proved partially effective. Treatment with anifrolumab, an IFNAR1 blocker, in conjunction with tocilizumab led to sustained suppression of IFN I scores, allowed discontinuation of JAKi, and resulted in significant improvement in vasculitic wounds.
Conclusion: This case underscores the challenges in treating patients with SAVI and highlights the utility of IFN I scores as a theragnostic biomarker. Although high-dose JAKi and dazukibart failed to achieve sustained control of IFN I signaling, treatment with anifrolumab durably suppressed IFN scores and demonstrated promising efficacy, which allows for the investigation of the role of IFN I signaling in the disease pathogenesis of SAVI and other interferonopathies in future clinical trials.
© 2025 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Cetin Gedik K, Souto Adeva G, Wade J, et al. Monitoring of BK reactivation and long‐term safety on JAK1/2 inhibition with baricitinib [abstract]. Arthritis Rheumatol 2020;72(suppl 10).
-
- U.S. Food and Drug Administration . “FDA requires warnings about increased risk of serious heart‐related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions.” U.S. Food and Drug Administration Drug Safety Communication, 1 Sept. 2021. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐requires‐warn....
-
- Neelakantan S, Oemar B, Johnson K, et al. Safety, tolerability, and pharmacokinetics of PF‐06823859, an anti‐Interferon β monoclonal antibody: a randomized, phase I, single‐ and multiple‐ascending‐dose study. Clin Pharmacol Drug Dev 2021;10(3):307–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 75N91019D00024/CA/NCI NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- Intramural Research Program at the National Institute of Allergy Immunology and Infectious Diseases of the National Institutes of Health
- 75N91019D000024/CA/NCI NIH HHS/United States
- 75N91019D000024/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

