Xanthine Derivative KMUP-3 Alleviates Periodontal Bone Resorption by Inhibiting Osteoclastogenesis and Macrophage Pyroptosis
- PMID: 40007249
- PMCID: PMC12371823
- DOI: 10.1111/jre.13393
Xanthine Derivative KMUP-3 Alleviates Periodontal Bone Resorption by Inhibiting Osteoclastogenesis and Macrophage Pyroptosis
Abstract
Aim: This study investigated the function effects of KMUP-3, a self-developed synthetic xanthine-based derivative, in suppressing Porphyromonas gingivalis (Pg-LPS)-aggravated osteoclastogenesis and pyroptosis as a potential treatment for periodontitis.
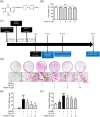
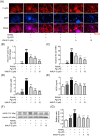
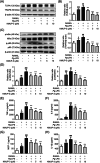
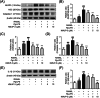
Methods: In vitro, the effects of Pg-LPS and KMUP-3 on osteoclast formation and macrophage pyroptosis were investigated using the receptor activator of nuclear factor-κB ligand (RANKL)-primed RAW264.7 macrophages. In vivo, the therapeutic effects of KMUP-3 were evaluated in a model of experimental periodontitis induced by gingival ligature placement.
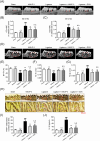
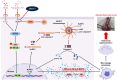
Results: We reveal that KMUP-3 suppressed osteoclastogenesis, inducible nitric oxide synthase activation, and reduced nitric oxide production enhanced by Pg-LPS in RANKL-primed RAW264.7 cells while also decreasing TLR4/NF-κB p65 pathway activation and decreased pro-inflammatory cytokine production; moreover, Pg-LPS promoted NLRP3 activation and exacerbated pyroptosis induction effects that were abolished by KMUP-3. Finally, KMUP-3 ameliorated alveolar bone loss and IL-1β levels in the gingival crevicular fluid in the rat ligature periodontitis model.
Conclusions: Our study demonstrated that KMUP-3 attenuates Pg-LPS-enhanced osteoclastogenesis and macrophage pyroptosis. Notably, KMUP-3 alleviates alveolar bone loss in experimental periodontitis rats and thus suggests its certain role in safeguarding against periodontal bone resorption.
Keywords: alveolar bone loss; nitric oxide; osteoclastogenesis; pyroptosis; xanthine derivative KMUP‐3.
© 2025 The Author(s). Journal of Periodontal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kassem A., Henning P., Lundberg P., Souza P. P., Lindholm C., and Lerner U. H., “ Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF‐κB Ligand) Through Activation of Toll‐Like Receptor 2 in Osteoblasts,” Journal of Biological Chemistry 290 (2015): 20147–20158, 10.1074/jbc.M115.655787. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

