Homologous recombination deficiency test validation in patients with high-grade advanced ovarian cancer
- PMID: 40007558
- PMCID: PMC11850238
- DOI: 10.3389/fmolb.2025.1524594
Homologous recombination deficiency test validation in patients with high-grade advanced ovarian cancer
Abstract
Background: Along with BRCA mutation status, homologous recombination deficiency (HRD) testing is a prognostic and predictive biomarker for poly-ADP-ribose polymerase (PARP) inhibitor therapy indication in high-grade epithelial ovarian, fallopian tube, or peritoneal cancer. Approximately 50% of high-grade serous ovarian cancers exhibit HRD, even in the absence of germline or somatic BRCA1/2 loss-of-function mutations. In this scenario, access to a validated diagnostic HRD test can optimize treatment selection and increase the effectiveness of the intervention.
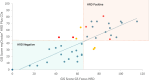
Objective: To technically validate an in-house next-generation sequencing (NGS)-based HRD test, QIAseq Custom Panel (QIAGEN), by comparing it with the reference assay, MyChoice CDx® Plus HRD (Myriad Genetics), which is used in routine care.
Methods: This is a prospective cohort study conducted at the Oncoclínicas Precision Medicine (OCPM) laboratory using samples from patients with advanced or relapsed platinum-sensitive ovarian cancer eligible for HRD testing in a diagnostic clinical setting at Oncoclínicas and Co. We assessed the performance of the in-house test (GS Focus HRD) using Cohen's kappa statistic to measure agreement with the gold standard assay (MyChoice® HRD Plus CDx) in HRD status classification, along with other accuracy metrics.
Results: In total, 41 samples were analyzed (20 HRD-positive, 19 HRD-negative, and 2 inconclusive results with the MyChoice® HRD Plus CDx assay). The GS Focus HRD test demonstrated high concordance for HRD status with the reference test (kappa: 0.8 and 95% CI: 0.60-0.98). Overall accuracy, sensitivity, and specificity were 90%. Six samples had BRCA1/2 mutations identified by the MyChoice® HRD Plus CDx, all of which were detected by the GS Focus HRD test.
Conclusion: In summary, the results demonstrate substantial agreement and high accuracy of the NGS-based GS Focus HRD test compared to MyChoice® HRD Plus CDx. Our in-house assay is eligible for diagnostic test approval and market access as per Brazilian regulations.
Keywords: BRCA1/2; homologous recombination deficiency; next-generation sequencing; ovarian cancer; validation.
Copyright © 2025 Nogueira Rodrigues, Souto, de Andrade, Gomes, Koide, e Silva, de Souza, Massaro, de Melo, Borges, Giro, de Andrade, da Costa, Gimenes, de Mello, de Oliveira, Lima, Lopes, Bretas, Jacob, Silva, Notaro, Alves, Moitinho, da Silva, Abramoff, Rauber, Dienstmann and Koyama.
Conflict of interest statement
Authors AN-R, AS, DdA, LG, SK, ReS, BdS, JM, AdM, AB, CG, CdA, CdC, DG, EdM, FdO, FL, GL, GB, GJ, HS, JN, LA, MM, MdS, RA, TA, RD, and FK were employed by Oncoclínicas and Co.
Figures
References
-
- Capoluongo E. D., Pellegrino B., Arenare L., Califano D., Scambia G., Beltrame L., et al. (2022). Alternative academic approaches for testing homologous recombination deficiency in ovarian cancer in the MITO16A/MaNGO-OV2 trial. ESMO Open 7 (5), 100585. 10.1016/j.esmoop.2022.100585 - DOI - PMC - PubMed
-
- Dexter J. M., Brubaker L. W., Bitler B. G., Goff B. A., Menon U., Moore K. N., et al. (2024). Ovarian cancer think tank: an overview of the current status of ovarian cancer screening and recommendations for future directions. Gynecol. Oncol. Rep. 53, 101376. 10.1016/j.gore.2024.101376 - DOI - PMC - PubMed
-
- Fountzilas E., Papadopoulou K., Chatzikonstantinou T., Karakatsoulis G., Constantoulakis P., Tsantikidi A., et al. (2023). Concordance between three homologous recombination deficiency (HRD) assays in patients with high-grade epithelial ovarian cancer. Cancers 15 (23), 5525. 10.3390/cancers15235525 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

