Diagnostic ultrasound enhances, then reduces, exogenously induced brain activity of mice
- PMID: 40007560
- PMCID: PMC11850526
- DOI: 10.3389/fnhum.2024.1509432
Diagnostic ultrasound enhances, then reduces, exogenously induced brain activity of mice
Abstract
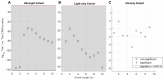
Transcranially delivered diagnostic ultrasound (tDUS) applied to the human brain can modulate those brains such that they became more receptive to external stimulation relative to sham ultrasound exposure. Here, we sought to directly measure the effect of tDUS on mouse brain activity subjected to an external stimulation-a blinking light. Using electrocorticography, we observed a substantial increase in median brain activity due to tDUS plus a blinking light relative to baseline and relative to sham tDUS plus a blinking light. Subsequent brain activity decreased after cessation of tDUS but with continuation of the blinking light, though it remained above that demonstrated by mice exposed to only a blinking light. In a separate experiment, we showed that tDUS alone, without a blinking light, had no observable effect on median brain activity, but upon its cessation, brain activity decreased. These results demonstrate that simultaneous exposure to tDUS and blinking light can increase the receptivity of the visual cortex of mice exposed to that light, and that prior exposure to tDUS can reduce subsequent brain activity. In each case, these results are consistent with published data. Our results on mice echo published human results but do not directly explain them, since their test subjects received less intense diagnostic ultrasound than did our mice. Given the near ubiquity of diagnostic ultrasound systems, further progress along this line of research could one day lead to the widespread use of diagnostic ultrasound to intentionally modulate human brain function during exogenous stimulation.
Keywords: LIFU; diagnostic ultrasound; electrocorticography; focused ultrasound; neuromodulation; ultrasound; ultrasound stimulation; visual stimulation.
Copyright © 2025 Tan, Griggs, Chen, Culevski, Floerchinger, Phutirat, Koh, Schimek and Mourad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Diagnosis of brain death by orbital Doppler ultrasound: a comparative research study.Turk Neurosurg. 2015;25(2):256-62. doi: 10.5137/1019-5149.JTN.8910-13.1. Turk Neurosurg. 2015. PMID: 26014009
-
Repeated Application of Transcranial Diagnostic Ultrasound Towards the Visual Cortex Induced Illusory Visual Percepts in Healthy Participants.Front Hum Neurosci. 2020 Mar 3;14:66. doi: 10.3389/fnhum.2020.00066. eCollection 2020. Front Hum Neurosci. 2020. PMID: 32194387 Free PMC article.
-
Transcranial Low-Intensity Focused Ultrasound Stimulation of the Visual Thalamus Produces Long-Term Depression of Thalamocortical Synapses in the Adult Visual Cortex.J Neurosci. 2024 Mar 13;44(11):e0784232024. doi: 10.1523/JNEUROSCI.0784-23.2024. J Neurosci. 2024. PMID: 38316559 Free PMC article.
-
Focused Ultrasound for Neuromodulation.Neurotherapeutics. 2019 Jan;16(1):88-99. doi: 10.1007/s13311-018-00691-3. Neurotherapeutics. 2019. PMID: 30488340 Free PMC article. Review.
-
Low Intensity Focused Ultrasound for Non-invasive and Reversible Deep Brain Neuromodulation-A Paradigm Shift in Psychiatric Research.Front Psychiatry. 2022 Feb 24;13:825802. doi: 10.3389/fpsyt.2022.825802. eCollection 2022. Front Psychiatry. 2022. PMID: 35280168 Free PMC article. Review.
References
-
- Bobola M. S., Chen L., Ezeokeke C. K., Olmstead T. A., Nguyen C., Sahota A., et al. . (2020). Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Aβ load chronically, as demonstrated in vivo. Brain Stimul. 13, 1014–1023. doi: 10.1016/j.brs.2020.03.016, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

