Biomaterials for neuroengineering: applications and challenges
- PMID: 40007617
- PMCID: PMC11855295
- DOI: 10.1093/rb/rbae137
Biomaterials for neuroengineering: applications and challenges
Abstract
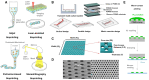
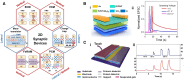
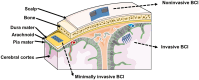
Neurological injuries and diseases are a leading cause of disability worldwide, underscoring the urgent need for effective therapies. Neural regaining and enhancement therapies are seen as the most promising strategies for restoring neural function, offering hope for individuals affected by these conditions. Despite their promise, the path from animal research to clinical application is fraught with challenges. Neuroengineering, particularly through the use of biomaterials, has emerged as a key field that is paving the way for innovative solutions to these challenges. It seeks to understand and treat neurological disorders, unravel the nature of consciousness, and explore the mechanisms of memory and the brain's relationship with behavior, offering solutions for neural tissue engineering, neural interfaces and targeted drug delivery systems. These biomaterials, including both natural and synthetic types, are designed to replicate the cellular environment of the brain, thereby facilitating neural repair. This review aims to provide a comprehensive overview for biomaterials in neuroengineering, highlighting their application in neural functional regaining and enhancement across both basic research and clinical practice. It covers recent developments in biomaterial-based products, including 2D to 3D bioprinted scaffolds for cell and organoid culture, brain-on-a-chip systems, biomimetic electrodes and brain-computer interfaces. It also explores artificial synapses and neural networks, discussing their applications in modeling neural microenvironments for repair and regeneration, neural modulation and manipulation and the integration of traditional Chinese medicine. This review serves as a comprehensive guide to the role of biomaterials in advancing neuroengineering solutions, providing insights into the ongoing efforts to bridge the gap between innovation and clinical application.
Keywords: 3D printing; biomaterials; brain-on-a-chip; nanopattern; neuroengineering; organoid.
© The Author(s) 2025. Published by Oxford University Press.
Figures









Similar articles
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
-
Innovative 3D bioprinting approaches for advancing brain science and medicine: a literature review.Biomed Phys Eng Express. 2024 Sep 25;10(6). doi: 10.1088/2057-1976/ad795c. Biomed Phys Eng Express. 2024. PMID: 39260389 Review.
-
Designs of Biomaterials and Microenvironments for Neuroengineering.Neural Plast. 2018 Dec 9;2018:1021969. doi: 10.1155/2018/1021969. eCollection 2018. Neural Plast. 2018. PMID: 30627148 Free PMC article. Review.
-
The Application of Biomaterial-Based Spinal Cord Tissue Engineering.Macromol Biosci. 2025 Mar;25(3):e2400444. doi: 10.1002/mabi.202400444. Epub 2024 Oct 29. Macromol Biosci. 2025. PMID: 39472074 Review.
-
Technological advances and challenges in constructing complex gut organoid systems.Front Cell Dev Biol. 2024 Aug 14;12:1432744. doi: 10.3389/fcell.2024.1432744. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39206092 Free PMC article. Review.
Cited by
-
Generation of Neural Organoids and Their Application in Disease Modeling and Regenerative Medicine.Adv Sci (Weinh). 2025 Aug;12(29):e01198. doi: 10.1002/advs.202501198. Epub 2025 May 24. Adv Sci (Weinh). 2025. PMID: 40411400 Free PMC article. Review.
References
-
- Micera S, Menciassi A, Cianferotti L, Gruppioni E, Lionetti V. Organ neuroprosthetics: connecting transplanted and artificial organs with the nervous system. Adv Healthc Mater 2024;13:e2302896. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

