Removal of Arsenic(V) from wastewater using calcined eggshells as a cost-effective adsorbent
- PMID: 40007776
- PMCID: PMC11850135
- DOI: 10.1016/j.heliyon.2025.e42505
Removal of Arsenic(V) from wastewater using calcined eggshells as a cost-effective adsorbent
Abstract
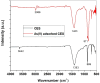
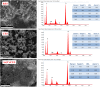
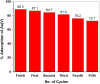
This study investigates calcined eggshells (CES) as an effective adsorbent for the remediation of As(V). Characterization of CES was performed using zeta potential analysis, FTIR, XRD and SEM-EDX. Batch studies were conducted to examine the effects of pH, adsorption kinetics, and adsorption isotherms to assess efficacy. The adsorption of As(V) followed the Langmuir isotherm and pseudo-second-order kinetics, with a maximum capacity of 91.05 mg g⁻1 at pH 6.0 and 298 K. The presence of additional anions such as chloride, sulfate, or nitrate had no significant impact on the biosorption of arsenate. However, the introduction of phosphate ions notably decreased the rate of arsenic adsorption. CES was easily regenerated with an alkaline solution and showed excellent reusability over four cycles. Thermodynamic studies confirmed the spontaneity and feasibility of the biosorption process. This study highlights that CES is a promising adsorbent for As(V) removal from contaminated water.
Keywords: Adsorption; Arsenate; Environmental applications; Wastewater treatment; Zeta potential.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Poudel B.R., Aryal R.L., Bhattarai S., Koirala A.R., Gautam S.K., Ghimire K.N., Pokhrel M.R. Agro-waste derived biomass impregnated with TiO2 as a potential adsorbent for removal of As(III) from water. Catalysts. 2020;10(10):1125. doi: 10.3390/catal10101125. - DOI
-
- World Health Organization (WHO): Geneva, Switzerland Arsenic in drinking-water background document for development of WHO guidelines for drinking-water quality. 2011. http://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf No. WHO/SDE/WSH/03.04/75/Rev/1); Available online:
LinkOut - more resources
Full Text Sources
Research Materials

