Dual-Responsive Methotrexate-Human Serum Albumin Complex-Encapsulated Liposomes for Targeted and Enhanced Atherosclerosis Therapy
- PMID: 40007906
- PMCID: PMC11853999
- DOI: 10.2147/IJN.S502850
Dual-Responsive Methotrexate-Human Serum Albumin Complex-Encapsulated Liposomes for Targeted and Enhanced Atherosclerosis Therapy
Abstract
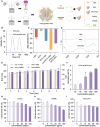
Introduction: In plaque sites of atherosclerosis (AS), the physiological barrier caused by the thick fiber cap due to the overmigration of vascular smooth muscle cells (VSMCs) prevents efficient drug delivery to damaged macrophages. How to ensure precise targeted delivery of drugs to plaque sites and their on-demand release to dysfunctional cells under the thick fibrous cap are feasible solutions to enhance AS treatment.
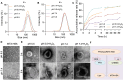
Methods: A small complex of methotrexate (MTX)-human serum albumin (HSA) with strong, thick fibrous cap penetration ability was encapsulated in a cholesterol hemisuccinate (CHEM) prepared pH-sensitive liposome, modifying with ROS-responsive PEG2000-TK-DSPE (PTD), termed PTD/Lipo/MTX-HSA.
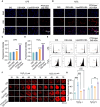
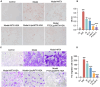
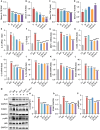
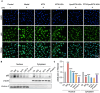
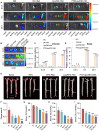
Results: PTD/Lipo/MTX-HSA can achieve precise targeting and on-demand release in response to plaques environments of AS. The designed formulation accelerated the release of the small-sized MTX-HSA complex in response to excess ROS and acidic pH conditions, and it better penetrated the macrophage spheroids. Furthermore, it has precise targeting ability in the AS mouse model and can produce good anti-inflammatory efficacy by inhibiting p65 entry into the nucleus turn out inflammatory factor.
Conclusion: Our formulations work with safety in mind, and it also highlights the potential of precisely targeted and on-demand-released dual-responsive smart nanoplatforms as promising therapeutic options to penetrate deeper plaques for the effective treatment of AS.
Keywords: dual-responsive smart liposomes; methotrexate-human serum albumin complex; penetration of deeper plaques.
© 2025 Wang et al.
Conflict of interest statement
The authors have declared no conflicts of interest in this work.
Figures
Similar articles
-
pH-responsive size-adjustable liposomes induce apoptosis of fibroblasts and macrophages for rheumatoid arthritis treatment.Acta Biomater. 2024 Apr 15;179:256-271. doi: 10.1016/j.actbio.2024.03.006. Epub 2024 Mar 12. Acta Biomater. 2024. PMID: 38484831
-
Macrophage Membrane-Cloaked ROS-Responsive Albumin Nanoplatforms for Targeted Delivery of Curcumin to Alleviate Acute Liver Injury.Mol Pharm. 2025 Feb 3;22(2):771-786. doi: 10.1021/acs.molpharmaceut.4c00808. Epub 2025 Jan 9. Mol Pharm. 2025. PMID: 39783460
-
Macrophage Membrane-Encapsulated Dopamine-Modified Poly Cyclodextrin Multifunctional Biomimetic Nanoparticles for Atherosclerosis Therapy.ACS Appl Mater Interfaces. 2024 Jun 26;16(25):32027-32044. doi: 10.1021/acsami.4c04431. Epub 2024 Jun 12. ACS Appl Mater Interfaces. 2024. PMID: 38867426
-
Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis.Biomaterials. 2017 Oct;143:93-108. doi: 10.1016/j.biomaterials.2017.07.035. Epub 2017 Jul 29. Biomaterials. 2017. PMID: 28778000
-
Surface saturation of drug-loaded hollow manganese dioxide nanoparticles with human serum albumin for treating rheumatoid arthritis.Drug Deliv. 2024 Dec;31(1):2380538. doi: 10.1080/10717544.2024.2380538. Epub 2024 Jul 23. Drug Deliv. 2024. PMID: 39044468 Free PMC article.
References
-
- Yuan J, Li L, Yang Q, et al. Targeted treatment of ischemic stroke by bioactive nanoparticle-derived reactive oxygen species responsive and inflammation-resolving nanotherapies. ACS Nano. 2021;15:16076. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

