Inhibition of Reaction Layer Formation on MgO(100) by Doping with Trace Amounts of Iron
- PMID: 40008203
- PMCID: PMC11848909
- DOI: 10.1021/acs.jpcc.4c06311
Inhibition of Reaction Layer Formation on MgO(100) by Doping with Trace Amounts of Iron
Abstract
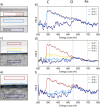
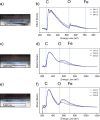
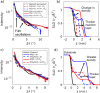
Despite extensive research on MgO's reactivity in the presence of CO2 under various conditions, little is known about whether impurities incorporated into the solid, such as iron, enhance or impede hydroxylation and carbonation reactions. The purity of the MgO required for the successful implementation of MgO looping as a direct air capture technology affects the deployment costs. With this motivation, we tested how incorporated iron impacts MgO (100) reactivity and passivation layer formation under ambient conditions by using atomic force microscopy, electron microscopy, and synchrotron-based X-ray scattering. Based on electron microprobe analysis, our MgO samples were 0.5 wt % iron, and Mössbauer spectroscopy results indicated that 70% of the iron is present as Fe(II). We find that even these low levels of iron dopants impeded both the hydroxylation at various relative humidities (10%, 33%, 75%, and >95%) and carbonation in CO2 (33%, 75%, and >95%) on the (100) surface. Crystalline reaction products were formed. Reaction layers on the sample were easily removed by exposing the sample to deionized water for 2 min. Overall, our findings demonstrate that the presence of iron dopants slows the reaction rate of MgO, indicating that MgO without incorporated iron is preferable for mineral looping applications.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Sievert K.; Schmidt T. S.; Steffen B. Considering technology characteristics to project future costs of direct air capture. Joule 2024, 8 (4), 979–999. 10.1016/j.joule.2024.02.005. - DOI
-
- Rausis K.; Stubbs A. R.; Power I. M.; Paulo C. Rates of atmospheric CO2 capture using magnesium oxide powder. Int. J. Greenhouse Gas Control. 2022, 119, 103701.10.1016/j.ijggc.2022.103701. - DOI
-
- Dostie L.; Rausis K.; Power I. M. Passive direct air capture using calcium oxide powder: The importance of water vapor. J. Cleaner Prod. 2024, 457, 142394.10.1016/j.jclepro.2024.142394. - DOI
LinkOut - more resources
Full Text Sources
