Publishers' Response to Post-Publication Concerns About Clinical Research in Women's Health
- PMID: 40008545
- PMCID: PMC12051221
- DOI: 10.1111/1471-0528.18100
Publishers' Response to Post-Publication Concerns About Clinical Research in Women's Health
Abstract
Objective: Potentially untrustworthy medical research is often identified after publication. We evaluated the effectiveness and efficiency of post-publication review of such studies in women's health.
Design: Cohort study.
Sample: Potentially untrustworthy papers published in women's health journals.
Methods: We wrote to the editors and publishers about potentially untrustworthy papers in women's health and requested an investigation according to the procedure established by the Committee of Publication Ethics (COPE).
Main outcome measure: Study characteristics, investigation outcome classed as retraction, expression of concern (EoC), correction or no wrongdoing found, and time to decision. We also report the case completion rate per journal and publisher.
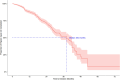
Results: Between 7th November 2017 and 30th April 2024, we wrote to editors and publishers of 891 potentially untrustworthy papers published in 206 different journals. At present, 263 (30%) of 891 papers received an outcome, with 227 (86%) labelled as problematic [152 (58%) retracted; 75 (29%) EoC]. For articles with a decision, it took a median time of 38 months for editors and publishers to decide, with 13% of the flagged cases reaching a decision within 12 months.
Conclusions: The current PPR process is inefficient and ineffective in assessing and removing untrustworthy data from the medical literature.
Keywords: Women's health; post‐publication review; trustworthiness; untrustworthy data.
© 2025 The Author(s). BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
B.W.M. is supported by a National Health Medical Research Council (NHMRC) Practitioner Fellowship (GNT1082548). B.W.M. reports consultancy, travel support and research funding from Merck and consultancy for Organon and Norgine.
Figures
Similar articles
-
Fate of articles that warranted retraction due to ethical concerns: a descriptive cross-sectional study.PLoS One. 2014 Jan 22;9(1):e85846. doi: 10.1371/journal.pone.0085846. eCollection 2014. PLoS One. 2014. PMID: 24465744 Free PMC article.
-
Improving biomedical journals' ethical policies: the case of research misconduct.J Med Ethics. 2014 Sep;40(9):644-6. doi: 10.1136/medethics-2013-101822. Epub 2014 Feb 6. J Med Ethics. 2014. PMID: 24505117
-
Reasons for retraction of clinical research articles in PubMed indexed medical journals from 2012 to 2022.Indian J Med Ethics. 2024 Oct-Dec;IX(4):296-300. doi: 10.20529/IJME.2024.067. Indian J Med Ethics. 2024. PMID: 39817295
-
Statement on Publication Ethics for Editors and Publishers.J Korean Med Sci. 2016 Sep;31(9):1351-4. doi: 10.3346/jkms.2016.31.9.1351. J Korean Med Sci. 2016. PMID: 27510376 Free PMC article. Review.
-
Research misconduct in health and life sciences research: A systematic review of retracted literature from Brazilian institutions.PLoS One. 2019 Apr 15;14(4):e0214272. doi: 10.1371/journal.pone.0214272. eCollection 2019. PLoS One. 2019. PMID: 30986211 Free PMC article.
References
-
- Carlisle J. B., “False Individual Patient Data and Zombie Randomised Controlled Trials Submitted to Anaesthesia,” Anaesthesia 76 (2021): 472–479. - PubMed
-
- Chambers L., Michener C., and Falcone T., “Plagiarism and Data Falsification Are the Most Common Reasons for Retracted Publications in Obstetrics and Gynaecology,” BJOG 126 (2019): 1134–1140. - PubMed
-
- Ioannidis J. P. A., “Hundreds of Thousands of Zombie Randomised Trials Circulate Among Us,” Anaesthesia 76 (2021): 444–447. - PubMed
-
- Saiz L. C., Erviti J., and Garjón J., “When Authors Lie, Readers Cry and Editors Sigh,” BMJ Evidence‐Based Medicine 23 (2018): 92–95. - PubMed
-
- Weeks J., Cuthbert A., and Alfirevic Z., “Trustworthiness Assessment as an Inclusion Criterion for Systematic Reviews—What Is the Impact on Results?,” Cochrane Evidence Synthesis and Methods 1 (2023): e12037.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous