Implementation strategies for WHO guidelines to prevent, detect, and treat postpartum hemorrhage
- PMID: 40008632
- PMCID: PMC11863301
- DOI: 10.1002/14651858.CD016223
Implementation strategies for WHO guidelines to prevent, detect, and treat postpartum hemorrhage
Abstract
Rationale: Despite World Health Organization (WHO) guidelines for preventing, detecting, and treating postpartum hemorrhage (PPH), effective implementation has lagged.
Objectives: To evaluate the clinical benefits and harms of implementation strategies used to promote adherence to WHO clinical guidelines for the prevention, detection, and treatment of PPH.
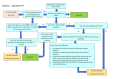
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, and two trial registries, along with reference checking, citation searching, and contact with study authors. The latest search date was 25 April 2024.
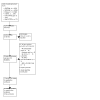
Eligibility criteria: We included randomized controlled trials (RCTs), including cluster, pragmatic, and stepped-wedge designs, and non-randomized studies of interventions (NRSIs), including interrupted time series (ITS) studies, controlled before-after (CBA) studies, and follow-up (cohort) studies containing concurrent controls that focused on or described implementation strategies of WHO guidelines for the prevention, detection, and treatment of PPH. Participants were birth attendants and people giving birth in a hospital or healthcare facility. We excluded studies that did not implement a WHO PPH recommendation, had no comparator group, or did not report clinical/implementation outcomes.
Outcomes: Our critical outcomes were: adherence to WHO-recommended guidelines for PPH prevention, detection, and treatment; PPH ≥ 500 mL; PPH ≥ 1000 mL; additional uterotonics within 24 hours after birth; blood transfusions; maternal death; severe morbidities (major surgery; admission to intensive care unit [ICU]); and adverse effects (variable and related to the clinical intervention) during hospitalization for birth. Our important outcomes were: breastfeeding at discharge; implementation outcomes such as acceptability, adoption, appropriateness, feasibility, fidelity, implementation cost, penetration, and sustainability of the implementation strategy; and health professional outcomes such as knowledge and skill.
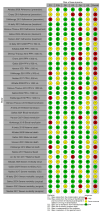
Risk of bias: We used the RoB 2 and ROBINS-I tools to assess risk of bias in RCTs and NRSIs, respectively.
Synthesis methods: Two review authors independently selected studies, performed data extraction, and assessed risk of bias and trustworthiness. Due to the nature of the data, we reported relevant results for each comparison and outcome but did not attempt quantitative synthesis. We used GRADE to assess the certainty of evidence.
Included studies: We included 13 studies (9 cluster-RCTs and 4 NRSIs) with a total of 1,027,273 births and more than 4373 birth attendants. The included studies were conducted in 17 different countries. Most trials were conducted in resource-limited settings. None of the included studies reported data on the use of additional uterotonics within 24 hours after birth or adverse effects.
Synthesis of results: Single-component implementation strategies versus usual care for PPH prevention, detection, and treatment We do not know if single-component implementation strategies have any effect on adherence to WHO PPH prevention recommendations, PPH ≥ 500 mL, PPH ≥ 1000 mL, or blood transfusion (very low-certainty evidence). Low-certainty evidence suggests that single-component implementation strategies may have little to no effect on maternal death (86,788 births, 3 trials); may increase severe morbidity related to ICU admission (26,985 births, 1 trial); and may reduce severe morbidity related to surgical outcomes (26,985 births, 1 trial). No trials in this comparison measured the effect on adherence to WHO treatment guidelines. Multicomponent implementation strategies versus usual care for PPH prevention, detection, and treatment We do not know if multicomponent implementation strategies have any effect on adherence to WHO PPH treatment recommendations, PPH ≥ 500 mL, blood transfusion, or severe morbidity relating to surgical outcomes (very low-certainty evidence). Multicomponent implementation strategies may have little to no effect on maternal death (274,008 births, 2 trials; low-certainty evidence) compared to usual care. No trials in this comparison measured the effect on adherence to WHO PPH prevention recommendations, PPH ≥ 1000 mL, or severe morbidity (outcomes related to ICU admission). Multicomponent implementation strategies versus enhanced usual care for PPH prevention, detection, and treatment Low-certainty evidence suggests that multicomponent implementation strategies may improve adherence to WHO PPH prevention recommendations (14,718 births, 2 trials) and adherence to WHO PPH treatment recommendations (356,913 births, 2 trials) compared to enhanced usual care. Multicomponent implementation strategies probably have little to no effect on maternal death (224,850 births, 2 trials; moderate-certainty evidence), severe morbidity related to ICU admission (224,850 births, 2 trials; moderate-certainty evidence), and surgical morbidity (210,132 births, 1 trial; moderate-certainty evidence) compared to enhanced usual care. We do not know if multicomponent implementation strategies affect PPH ≥ 500 mL, PPH ≥ 1000 mL, or blood transfusion (very low-certainty evidence).
Authors' conclusions: Multicomponent implementation strategies may improve adherence to WHO PPH prevention and treatment recommendations, but they probably result in little to no difference in ICU admissions, surgical morbidity, or maternal death. The majority of available evidence is of low to very low certainty, thus we cannot draw any robust conclusions on the effects of implementation strategies for WHO guidelines to prevent, detect, and treat PPH. While all included studies used the implementation strategy of 'train and educate,' the effects seem to be limited when used as a single strategy. Additional research using pragmatic, hybrid effectiveness-implementation study designs that measure implementation outcomes simultaneously alongside clinical outcomes would be beneficial to understand contextual factors, barriers, and facilitators that affect implementation.
Funding: This Cochrane review had no dedicated external funding. Dr Rose Molina, who is employed by Beth Israel Deaconess Medical Center, received funding from Ariadne Labs (Harvard T.H. Chan School of Public Health, Brigham and Women's Hospital) for her time. As a funder, Ariadne Labs had no involvement in the development of the protocol or conduct of the review. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of Ariadne Labs.
Registration: Registration: PROSPERO (CRD42024563802) available via https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024563802.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
KS: Brigham and Women's Hospital (Employment); Bill and Melinda Gates Foundation (Grant/Contract)—funding of the BetterBirth trial (The WHO Safe Childbirth Checklist Trial; NCT02148952), grant #OPP1017378; co‐principal investigator/Scientific Director of the BetterBirth trial—this study did not appear in our literature search and thus was not captured by our current review; however, it could become eligible for future updates depending on search parameters. If the BetterBirth trial is included in future review version(s), KS will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for the BetterBirth trial.
EL: no relevant interests; Clinical Fellow at Beth Israel Deaconess Medical Center.
RM: no relevant interests; Obstetrician‐Gynecologist, Beth Israel Deaconess Medical Center. If RM becomes involved in any study that could be included in future update(s) of this review, RM will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for that study.
MMD: Harvard University School of Public Health (Employment); involved in the BetterBirth trial (The WHO Safe Childbirth Checklist trial; NCT02148952), funded by the Bill and Melinda Gates Foundation (grant #OPP1017378)—this study did not appear in our literature search and thus was not captured by our current review; however, it could become eligible for future update(s) of this review depending on search parameters. If the BetterBirth trial is included in future review version(s), MMD will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for the BetterBirth trial.
LC: no relevant interests; Evidence Synthesis Development Editor, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
LR: no relevant interests; Evidence Synthesis Development Editor, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
ANS: no relevant interests; Information Specialist, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
JMG: no relevant interests.
Figures
References
-
- World Health Organization. A Roadmap to Combat Postpartum Haemorrhage Between 2023 and 2030. Geneva: World Health Organization, 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous