Mapping methods gaps between EU joint clinical assessments and local health technology assessment decision-making: an environmental scan of guidance in select EU markets and harmonization challenges
- PMID: 40008768
- PMCID: PMC12007483
- DOI: 10.57264/cer-2024-0240
Mapping methods gaps between EU joint clinical assessments and local health technology assessment decision-making: an environmental scan of guidance in select EU markets and harmonization challenges
Abstract
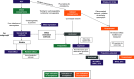
Aim: Under the newly instituted health technology assessment (HTA) regulation (HTAR), health technology developers must build evidence packages that meet the needs for both the upcoming EU joint clinical assessment (JCA) and national decision-making. In-depth knowledge of local methodological requirements as well as preparedness for effective strategic development is crucial. This study aimed to review methodological guidance documents to map similarities/misalignments between the EU HTAR and select HTA agencies. Materials & methods: An environmental scan was performed in March 2024 and updated in December 2024 of the websites for European Network for HTA, the European Commission and HTA agencies in France, Germany, The Netherlands and Spain. The search aimed to systematically identify and summarize methodological guidance documents from the respective organizations on scoping considerations, evidence identification and synthesis. Results: Overall, published EU HTAR methods guidelines are detailed, prescriptive and make reference to a preference (or lack thereof) for specific analytical methods. There was consensus among EU JCA and local HTA guidelines that clinical comparative assessments should be based on a systematically identified, unbiased selected evidence base derived from various sources. However, agencies differed on guidance related to evidence derived from indirect treatment comparisons. Conclusion: An environmental scan of methods documents revealed that it will likely be challenging for health technology developers to build strong evidence packages that can support both EU JCA and local reimbursement decision-making. A greater understanding of the similarities and differences between EU and local HTA requirements will be needed, including a greater capacity to demonstrate value through advanced analytics.
Keywords: European Union; comparative effectiveness; data; health technology assessment; joint clinical assessment; methods; uncertainty.
Conflict of interest statement
Competing interests disclosure
All authors are employees of Cytel, Inc. The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


Similar articles
-
Advancing cooperation in Health Technology Assessment in Europe: insights from the EUnetHTA 21 project amidst the evolving legal landscape of European HTA.Int J Technol Assess Health Care. 2024 Dec 12;40(1):e75. doi: 10.1017/S0266462324004689. Int J Technol Assess Health Care. 2024. PMID: 39663913 Free PMC article.
-
Health technology assessment of medical devices: What is different? An overview of three European projects.Z Evid Fortbild Qual Gesundhwes. 2015;109(4-5):309-18. doi: 10.1016/j.zefq.2015.06.011. Epub 2015 Jul 26. Z Evid Fortbild Qual Gesundhwes. 2015. PMID: 26354131 Review.
-
The maze of real-world evidence frameworks: from a desert to a jungle! An environmental scan and comparison across regulatory and health technology assessment agencies.J Comp Eff Res. 2024 Sep;13(9):e240061. doi: 10.57264/cer-2024-0061. Epub 2024 Aug 12. J Comp Eff Res. 2024. PMID: 39132748 Free PMC article.
-
Health technology assessment in Europe: A comparison of organizations and introduction to the European regulation.Presse Med. 2025 Jun;54(2):104282. doi: 10.1016/j.lpm.2025.104282. Epub 2025 Apr 7. Presse Med. 2025. PMID: 40204061
-
Policies for Use of Real-World Data in Health Technology Assessment (HTA): A Comparative Study of Six HTA Agencies.Value Health. 2017 Apr;20(4):520-532. doi: 10.1016/j.jval.2016.12.003. Epub 2017 Jan 27. Value Health. 2017. PMID: 28407993 Review.
References
-
- European Commission. Implementing Regulation (EU) 2024/1381 on joint clinical assessment of medicinal products for human use. (2024). Date Accessed: 18 December 2024. https://health.ec.europa.eu/publications/implementing-regulation-eu-2024...
-
• Critical as describes the principles of EU health technology assessment regulation (HTAR).
-
- European Commission. Health technology assessment: overview. Date Accessed: 17 December 2024. https://health.ec.europa.eu/health-technology-assessment/overview_en
-
- O'Rourke B, Oortwijn W, Schuller T. Announcing the new definition of health technology assessment. Value Health 23(6), 824–825 (2020). - PubMed
-
- O'Rourke B, Oortwijn W, Schuller T et al. The new definition of health technology assessment: a milestone in international collaboration. Int. J. Technol. Assess. Health Care 36(3), 187–190 (2020). - PubMed
-
- Kristensen FB, Lampe K, Wild C et al. The HTA Core Model((R))-10 years of developing an international framework to share multidimensional value assessment. Value Health 20(2), 244–250 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
