Plasma p-tau217 identifies cognitively normal older adults who will develop cognitive impairment in a 10-year window
- PMID: 40008832
- PMCID: PMC11863240
- DOI: 10.1002/alz.14537
Plasma p-tau217 identifies cognitively normal older adults who will develop cognitive impairment in a 10-year window
Abstract
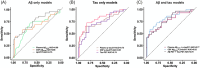
Introduction: We assessed the prognostic accuracy of plasma p-tau217 in predicting the progression to mild cognitive impairment (MCI) in cognitively unimpaired (CU) individuals over a mean follow-up of 5.65 years after plasma collection (range 1.01-10.47).
Methods: We included 215 participants from the PREVENT-AD cohort with plasma Aβ42/40 and p-tau217, 159 with cerebrospinal fluid (CSF) Aβ42/40 and p-tau217, and 155 with 18F-NAV4694 and 18F-flortaucipir PET scans. MCI progression was determined by multidisciplinary consensus among memory experts blind to biomarker and genetic information.
Results: Cox proportional hazard models indicated a greater progression rate in A+T+plasma and A-T+plasma compared to A-T-plasma individuals (HR = 7.81 [95% CI = 3.92 to 15.59] and HR = 4.25 [1.60-11.31] respectively). Similar results were found with CSF (HR = 3.63 [1.72-7.70]) and PET (HR = 9.30 [3.67-23.55]).
Discussion: Plasma p-tau217 is a prognostic marker for identifying individuals who will develop cognitive impairment within ten years.
Highlights: Elevated plasma p-tau217 levels in CU individuals indicate future clinical progression. Adding plasma Aβ42/40 status to p-tau markers did not improve the prediction to MCI. All individuals with abnormal tau PET measured in a temporal meta-ROI progressed to MCI.
Keywords: CSF; MCI; PET; amyloid; plasma; tau.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). M.S. has served on advisory boards for Roche, Novo Nordisk, and Servier; received speaker honoraria from Bioarctic, Eisai, Genentech, Novo Nordisk, and Roche; and receives research support (to the institution) from Alzpath, Bioarctic, Novo Nordisk, and Roche (outside scope of submitted work). He is a co‐founder of Centile Bioscience Ltd. No other disclosures were reported. Author disclosures are available in the supporting information.
Figures


References
-
- Strikwerda−Brown C, Hobbs DA, Gonneaud J, et al. Association of elevated amyloid and tau positron emission tomography signal with near‐term development of Alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurol. 2022;79(10):975‐985. doi:10.1001/jamaneurol.2022.2379 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- #ADSF-21-831377-C/ALZ/Alzheimer's Association/United States
- #2023-06188/Swedish Research Council
- the Stiftelsen för Gamla Tjänarinnor
- Sahlgrenska Academy at the University of Gothenburg
- VGFOUREG-995510/Västra Götaland Region R&D
- Fondation Brain Canada
- #2021-02678/Swedish Research Council
- Biogen Canada
- AF-994900/the Alzheimerfonden
- 860197/European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement
- KAW2014.0363/Knut och Alice Wallenbergs Stiftelse
- 101112145/HORIZON EUROPE Reforming and enhancing the European Research and Innovation system
- the Fonds de recherche du Québec
- R01 AG081394/AG/NIA NIH HHS/United States
- #2017-02869/Swedish Research Council
- #ADSF-21-831376-C/ALZ/Alzheimer's Association/United States
- Familjen Rönströms Stiftelse
- the Canadian Institutes of Health Research
- #201809-2016862/Alzheimer's Drug Discovery Foundation
- and #ADSF-24-1284328-C/ALZ/Alzheimer's Association/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- the Cure Alzheimer's Fund
- R01AG081394-01/the National Institute of Health
- Erling-Persson Family Foundation
- #ALFGBG-965326/Swedish State Support for Clinical Research
- UKDRI-1003/UK Dementia Research Institute at UCL
- Bluefield Project
- KAW 2023.0371/Knut och Alice Wallenbergs Stiftelse
- Fondation Jean-Louis Lévesque
- #ADSF-21-831381-C/ALZ/Alzheimer's Association/United States
- #ALFGBG-71320/Swedish State Support for Clinical Research
- 101132933/HORIZON EUROPE Reforming and enhancing the European Research and Innovation system
- Natural Sciences and Engineering Research Council of Canada
- the Hjärnfonden
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- #2021-06545/Swedish Research Council
- #ALFGBG813971/Swedish State Support for Clinical Research
- National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre
LinkOut - more resources
Full Text Sources
Medical

