Evaluation of Electrospun Poly-4-Hydroxybutyrate as Biofunctional and Degradable Scaffold for Pelvic Organ Prolapse in a Vaginal Sheep Model
- PMID: 40008865
- PMCID: PMC11995834
- DOI: 10.1002/mabi.202400412
Evaluation of Electrospun Poly-4-Hydroxybutyrate as Biofunctional and Degradable Scaffold for Pelvic Organ Prolapse in a Vaginal Sheep Model
Abstract
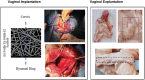
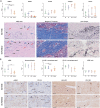
Pelvic organ prolapse (POP) affects many women, especially after menopause. POP occurs due to the descent of weakened supportive tissue. Current prolapse surgeries have high failure rates, due to disturbed wound healing caused by lower tissue regeneration and estrogen depletion. Absorbable poly-4-hydroxybutyrate (P4HB) knit implants exhibited improved cell and tissue response leading to less complications from prolapse surgery. This study aims to enhance wound healing and improve surgical outcomes by using an electrospun (ES) P4HB scaffold (ES P4HB) that emulates natural tissue structure. Further 17β-estradiol (E2)-a prominent wound healing factor-is incorporated into the scaffold (ES P4HB-E2). Parous Dohne Merino sheep underwent posterior vaginal wall implantation of either P4HB (n = 6) or 17β-estradiol relasing P4HB-E2 (n = 6) scaffolds, or underwent native tissue repair (NTR) (n = 4). Vaginal explants were compared for short-term host response in terms of gross necropsy, histomorphology, biomechanics, tissue-integration, and degradation of P4HB at 3-months post-implantation. Both scaffolds show promising results with enhanced mechanical properties and increased macrophage infiltration compared to NTR, but without differences between scaffolds. Thus, it seems electrospun P4HB scaffolds already improve tissue integration and healing. Further long-term studies are needed before these scaffolds can be used in clinical practice.
Keywords: absorbable scaffold; electrospinning; estradiol; poly‐4‐hydroxybutyrate (P4HB); vaginal sheep model.
© 2025 The Author(s). Macromolecular Bioscience published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Brito L. G. O., Pereira G. M. V., Moalli P., Shynlova O., Manonai J., Weintraub A. Y., Deprest J., Bortolini M. A. T., Int. Urogynecol. J. 2022, 33, 15. - PubMed
-
- Deprest J. A., Cartwright R., Dietz H. P., Brito L. G. O., Koch M., Allen‐Brady K., Manonai J., Weintraub A. Y., Chua J. W. F., Cuffolo R., Sorrentino F., Cattani L., Decoene J., Page A.‐S., Weeg N., Varella Pereira G. M., Mori da Cunha de Carvalho M. G. M. C., Mackova K., Hympanova L. H., Moalli P., Shynlova O., Alperin M., Bortolini M. A. T., Int. Urogynecol. J. 2022, 33, 1699. - PubMed
-
- Smith F. J., Holman C. D., Moorin R. E., Tsokos N., Obstet. Gynecol. 2010, 116, 1096. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

