Antibodies with specificity to glycan motifs that decorate OMV cargo proteins
- PMID: 40008882
- PMCID: PMC11934327
- DOI: 10.1128/msphere.00907-24
Antibodies with specificity to glycan motifs that decorate OMV cargo proteins
Abstract
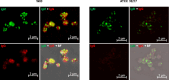
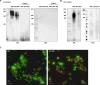
Porphyromonas gingivalis is a major etiological agent of periodontal disease, and infections with this bacterium are associated with systemic pathologies, including atherosclerosis, rheumatoid arthritis, and Alzheimer's disease. P. gingivalis has a variety of immune evasion mechanisms and exhibits highly variable cell surface characteristics that are strain dependent, complicating the development of effective vaccines and therapeutics. Here, we show that a subset of immunoglobulin M (IgM) antibodies in antiserum raised against P. gingivalis strain W83 selectively recognize the outer membrane vesicles (OMVs). Pre-adsorption with a mutant strain lacking an OMV-specific lipoprotein (PG1881) that has been shown to be glycosylated significantly enhanced IgM specificity toward PG1881 and the OMVs. In addition, the IgM reactivity against the OMVs derived from a mutant lacking enzymes required for O-glycosylation was markedly reduced, indicating that the IgM targets the glycan motifs on proteins carried on OMVs. Importantly, the IgM exhibited specific recognition of OMVs from both P. gingivalis and Porphyromonas endodontalis, while showing low reactivity toward other genera belonging to the phylum Bacteroidetes. This study revealed a potential host evasion strategy and highlights the potential for utilizing O-glycans in vaccine development and OMV-targeted antibodies in therapeutic interventions to combat P. gingivalis infections.
Importance: O-glycosylation of cell surface proteins by bacteria is known to play a role in various functions including colonization and immune evasion. This study highlights the identification of IgM antibodies that specifically recognize O-glycosylated proteins that are selectively carried on outer membrane vesicles (OMVs). The findings suggest a potential host evasion mechanism and open new avenues for using OMVs in vaccine development and targeting O-glycans with antibodies as a therapeutic strategy against the subgingival pathobiont P. gingivalis.
Keywords: IgM antibodies; O-glycosylation; OMV; P. gingivalis; PG1881.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Waller T, Kesper L, Hirschfeld J, Dommisch H, Kölpin J, Oldenburg J, Uebele J, Hoerauf A, Deschner J, Jepsen S, Bekeredjian-Ding I. 2016. Porphyromonas gingivalis outer membrane vesicles induce selective tumor necrosis factor tolerance in a toll-like receptor 4- and mTOR-dependent manner. Infect Immun 84:1194–1204. doi:10.1128/IAI.01390-15 - DOI - PMC - PubMed
-
- Fleetwood AJ, Lee MKS, Singleton W, Achuthan A, Lee MC, O’Brien-Simpson NM, Cook AD, Murphy AJ, Dashper SG, Reynolds EC, Hamilton JA. 2017. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol 7:351. doi:10.3389/fcimb.2017.00351 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01DE031159/HHS | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- R01DE033659/HHS | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- NRF-2022R1C1C2007752/National Research Foundation of Korea (NRF)
- R01 DE031159/DE/NIDCR NIH HHS/United States
- R01 DE019117/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
