Cell differentiation controls iron assimilation in the choanoflagellate Salpingoeca rosetta
- PMID: 40008892
- PMCID: PMC11934334
- DOI: 10.1128/msphere.00917-24
Cell differentiation controls iron assimilation in the choanoflagellate Salpingoeca rosetta
Abstract
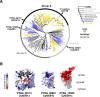
Marine microeukaryotes have evolved diverse cellular features that link their life histories to surrounding environments. How those dynamic life histories intersect with the ecological functions of microeukaryotes remains a frontier to understanding their roles in critical biogeochemical cycles. Choanoflagellates, phagotrophs that cycle nutrients through filter feeding, provide models to explore this intersection, for many choanoflagellate species transition between life history stages by differentiating into distinct cell types. Here, we report that cell differentiation in the marine choanoflagellate Salpingoeca rosetta endows one of its cell types with the ability to utilize insoluble ferric colloids. These colloids are a predominant form of iron in marine environments and are largely inaccessible to cell-walled microbes. Therefore, choanoflagellates and other phagotrophic eukaryotes may serve critical ecological roles by cycling this essential nutrient through iron utilization pathways. We found that S. rosetta can utilize these ferric colloids via the expression of a cytochrome b561 iron reductase (cytb561a). This gene and its mammalian ortholog, the duodenal cytochrome b561 (DCYTB) that reduces ferric cations for uptake in gut epithelia, belong to a subgroup of cytochrome b561 proteins with distinct biochemical features that contribute to iron reduction activity. Overall, our findings provide insight into the ecological roles choanoflagellates perform and inform reconstructions of early animal evolution where functionally distinct cell types became an integrated whole at the origin of animal multicellularity.
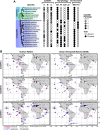
Importance: This study examines how cell differentiation in a choanoflagellate enables the uptake of iron, an essential nutrient. Choanoflagellates are widespread, aquatic microeukaryotes that are the closest living relatives of animals. Similar to their animal relatives, we found that the model choanoflagellate, S. rosetta, divides metabolic functions between distinct cell types. One cell type uses an iron reductase to acquire ferric colloids, a key source of iron in the ocean. We also observed that S. rosetta has three variants of this reductase, each with distinct biochemical properties that likely lead to differences in how they reduce iron. These reductases are variably distributed across ocean regions, suggesting a role for choanoflagellates in cycling iron in marine environments.
Keywords: cell-type evolution; choanoflagellate; cytochrome b561; iron colloid; marine microeukaryote.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Cell differentiation controls iron assimilation in a choanoflagellate.bioRxiv [Preprint]. 2024 Sep 16:2024.05.25.595918. doi: 10.1101/2024.05.25.595918. bioRxiv. 2024. Update in: mSphere. 2025 Mar 25;10(3):e0091724. doi: 10.1128/msphere.00917-24. PMID: 39345370 Free PMC article. Updated. Preprint.
References
-
- González JM, Suttle CA. 1993. Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Mar Ecol Prog Ser 94:1–10. doi:10.3354/meps094001 - DOI
-
- Barbeau K, Moffett JW, Caron DA, Croot PL, Erdner DL. 1996. Role of protozoan grazing in relieving iron limitation of phytoplankton. Nature 380:61–64. doi:10.1038/380061a0 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
