Posterior cingulate cortex microRNA dysregulation differentiates cognitive resilience, mild cognitive impairment, and Alzheimer's disease
- PMID: 40008917
- PMCID: PMC11863362
- DOI: 10.1002/alz.70019
Posterior cingulate cortex microRNA dysregulation differentiates cognitive resilience, mild cognitive impairment, and Alzheimer's disease
Abstract
Introduction: MicroRNA (miRNA) activity is increasingly appreciated as a key regulator of pathophysiologic pathways in Alzheimer's disease (AD). However, the role of miRNAs during the progression of AD, including resilience and prodromal syndromes such as mild cognitive impairment (MCI), remains underexplored.
Methods: We performed miRNA-sequencing on samples of posterior cingulate cortex (PCC) obtained post mortem from Rush Religious Orders Study participants diagnosed ante mortem with no cognitive impairment (NCI), MCI, or AD. NCI subjects were subdivided as low pathology (Braak stage I/II) or high pathology (Braak stage III/IV), suggestive of resilience. Bioinformatics approaches included differential expression, messenger RNA (mRNA) target prediction, interactome modeling, functional enrichment, and AD risk modeling.
Results: We identified specific miRNA groups, mRNA targets, and signaling pathways distinguishing AD, MCI, resilience, ante mortem neuropsychological test performance, post mortem neuropathological burden, and AD risk.
Discussion: These findings highlight the potential of harnessing miRNA activity to manipulate disease-modifying pathways in AD, with implications for precision medicine.
Highlights: MicroRNA (MiRNA) dysregulation is a well-established feature of Alzheimer's disease (AD). Novel miRNAs also distinguish subjects with mild cognitive impairment and putative resilience. MiRNAs correlate with cognitive performance and neuropathological burden. Select miRNAs are associated with AD risk with age as a significant covariate. MiRNA pathways include insulin, prolactin, kinases, and neurite plasticity.
Keywords: dementia; microRNA; mild cognitive impairment; non‐coding RNA; resilience.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no competing interests. Author disclosures are available in the supporting information.
Figures



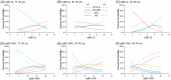
 ), NCI‐HP (
), NCI‐HP ( ), MCI (
), MCI ( ), and AD (
), and AD ( ) at ages 75–85 (A,D), 85–90 (B,E), and 90–96 (C,F). AD, Alzheimer's disease; MCI, mild cognitive impairment; miRNAs, microRNAs; NCI‐HP, no cognitive impairment patients with high AD pathology; NCI‐LP, no cognitive impairment patients with low AD pathology.
) at ages 75–85 (A,D), 85–90 (B,E), and 90–96 (C,F). AD, Alzheimer's disease; MCI, mild cognitive impairment; miRNAs, microRNAs; NCI‐HP, no cognitive impairment patients with high AD pathology; NCI‐LP, no cognitive impairment patients with low AD pathology.References
MeSH terms
Substances
Grants and funding
- AG074004/AG/NIA NIH HHS/United States
- AG077103/AG/NIA NIH HHS/United States
- R56 AG072599/AG/NIA NIH HHS/United States
- AG017617/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- AG014449/AG/NIA NIH HHS/United States
- R01 AG074004/AG/NIA NIH HHS/United States
- the Arizona Alzheimer's Consortium at Barrow Neurological Institute
- R01 AG072599/AG/NIA NIH HHS/United States
- R01 AG085572/AG/NIA NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- AG072599/AG/NIA NIH HHS/United States
- P30 AG072975/NH/NIH HHS/United States
- AG081286/AG/NIA NIH HHS/United States
- RF1 AG077103/AG/NIA NIH HHS/United States
- AG072931/AG/NIA NIH HHS/United States
- Indiana Alzheimer's Disease Research Center
- P30 AG072976/AG/NIA NIH HHS/United States
- P01 AG017617/AG/NIA NIH HHS/United States
- P30 AG072931/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

