Elucidating Gas Reduction Effects of Organosilicon Additives in Lithium-Ion Batteries
- PMID: 40008987
- PMCID: PMC11912472
- DOI: 10.1021/jacs.5c00402
Elucidating Gas Reduction Effects of Organosilicon Additives in Lithium-Ion Batteries
Abstract
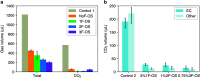
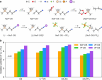
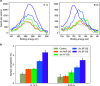
Lithium-ion batteries (LIBs) with nonaqueous liquid electrolytes are prone to gas generation at elevated voltages and temperatures, degrading battery performance and posing serious safety risks. Organosilicon (OS) additives are an emerging candidate solution for gassing problems in LIBs, but a detailed understanding of their functional mechanisms remains elusive. In this work, we present a combined computational and experimental study to elucidate the gas-reducing effects of OS additives. Cell volume measurements and gas chromatography-mass spectrometry reveal that OS additives can substantially reduce gas evolution in LIBs, particularly CO2 regardless of source. Through density functional theory calculations, we identify multiple plausible pathways for CO2 evolution, including (1) nucleophile-induced ring-opening of ethylene carbonate (EC) and the subsequent electro-oxidation and (2) direct electro-oxidation of lithium carbonate (Li2CO3). Correspondingly, we find that OS additives function via two primary mechanisms: (1) scavenging of nucleophiles such as superoxide (O2•-), peroxide (O22-), and carbonate ion (CO32-); (2) oligomerization with ethylene carbonate oxide ion and ethylene dicarbonate ion. Moreover, we discover that OS additives possess strong lithium coordination affinity, which helps further reduce the nucleophilic reaction energies and hence increases their nucleophile-scavenging efficiency. Finally, we provide a mechanistic interpretation for the enhanced gas-reduction effects observed with fluorinated OS compounds, corroborated by surface analysis results from X-ray photoelectron spectroscopy. Our study offers the first molecular-level insights into how OS additives contribute to reduced gas formation in LIBs, paving the way for improved safety and performance of LIBs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Aurbach D.; Talyosef Y.; Markovsky B.; Markevich E.; Zinigrad E.; Asraf L.; Gnanaraj J. S.; Kim H.-J. Design of Electrolyte Solutions for Li and Li-Ion Batteries: A Review. Electrochim. Acta 2004, 50 (2–3), 247–254. 10.1016/j.electacta.2004.01.090. - DOI
-
- Scrosati B.; Hassoun J.; Sun Y.-K. Lithium-ion batteries. A Look into the Future. Energy Environ. Sci. 2011, 4 (9), 3287.10.1039/c1ee01388b. - DOI

