Cytochromes P460 and c'-β: exploiting a novel fold for multiple functions
- PMID: 40009202
- PMCID: PMC11928373
- DOI: 10.1007/s00775-025-02102-3
Cytochromes P460 and c'-β: exploiting a novel fold for multiple functions
Abstract
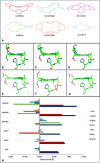
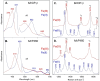
Two related classes of ligand-binding heme c-containing proteins with a high degree of structural homology have been identified and characterized over recent decades: cytochromes P460 (cyts P460), defined by an unusual heme-lysine cross-link, and cytochromes c'-β (cyts c'-β), containing a canonical c-heme without the lysine cross-link. The shared protein fold of the cyt P460-cyt c'-β superfamily can accommodate a variety of heme environments with entirely different reactivities. On the one hand, cyts P460 with polar distal pockets have been shown to oxidize NH2OH to NO and/or N2O via proton-coupled electron transfer. On the other hand, cyts c'-β with hydrophobic distal pockets have a proposed gas binding function similar to the unrelated, but more extensively characterized, alpha helical cytochromes c'. Recent studies have also identified 'halfway house' proteins (cyts P460 with non-polar heme pockets and cyts c'-β with polar distal heme pockets) with functions yet to be resolved. Here, we review the structural, spectroscopic and enzymatic properties of the cyt P460-cyt c'-β superfamily with a view to understanding the structural determinants of their different functional properties.
Keywords: Cross-link; Gas binding; Haem; Heme; Nitrification; P460.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no competing interests. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable.
Figures





References
-
- Paoli M, Marles-Wright J, Smith A (2002) Structure-function relationships in heme-proteins. DNA Cell Biol 21:271–280. 10.1089/104454902753759690 - PubMed
-
- Mense SM, Zhang L (2006) Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res 16:681–692. 10.1038/sj.cr.7310086 - PubMed
-
- Zahn JA, Arciero DM, Hooper AB, Dispirito AA (1996) Cytochrome c′ of Methylococcus capsulatus Bath. Eur J Biochem 240:684–691. 10.1111/j.1432-1033.1996.0684h.x - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

