STING activation improves T-cell-engaging immunotherapy for acute myeloid leukemia
- PMID: 40009483
- PMCID: PMC12105722
- DOI: 10.1182/blood.2024026934
STING activation improves T-cell-engaging immunotherapy for acute myeloid leukemia
Abstract
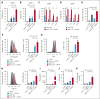
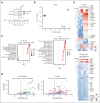
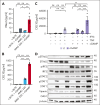
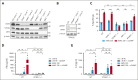
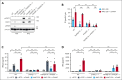
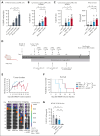
T-cell-recruiting bispecific antibodies (BsAbs) are in clinical development for relapsed/refractory acute myeloid leukemia (AML). Despite promising results, early clinical trials have failed to demonstrate durable responses. We investigated whether activation of the innate immune system through stimulator of interferon (IFN) genes (STING) can enhance target cell killing by a BsAb targeting CD33 (CD33 bispecific T-cell engager molecule; AMG 330). Indeed, we show that cytotoxicity against AML mediated by AMG 330 can be greatly enhanced when combined with the STING agonist 2',3'-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) or diamidobenzimidazole (diABZI). We used in vitro cytotoxicity assays, immunoblotting, transcriptomic analyses, and extensive CRISPR-Cas9 knockout experiments to investigate the enhancing effect of a STING agonist on the cytotoxicity of AMG 330 against AML. Importantly, we validated our findings with primary AML cells and in a xenograft AML model. Mechanistically, in addition to direct cytotoxic effects of STING activation on AML cells, activated T cells render AML cells more susceptible to STING activation through their effector cytokines, IFN-γ and tumor necrosis factor, resulting in enhanced type I IFN production and induction of IFN-stimulated genes. This feeds back to the T cells, leading to a further increase in effector cytokines and an overall cytotoxic T-cell phenotype, contributing to the beneficial effect of cGAMP/diABZI in enhancing AMG 330-mediated lysis. We established a key role for IFN-γ in AMG 330-mediated cytotoxicity against AML cells and in rendering AML cells responsive to STING agonism. Here, we propose to improve the efficacy of CD33-targeting BsAbs by combining them with a STING agonist.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. receives industry research support from Amgen, Bristol Myers Squibb (BMS)/Celgene, Gilead/Kite, Johnson & Johnson, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, and Takeda; and serves as a consultant/advisor for AbbVie, Crossbow, Debiopharm, Gilead/Kite, Interius, Johnson & Johnson, Molecular Partners, Novartis, and Otsuka; and serves on the speakers’ bureau at Amgen, BMS/Celgene, Gilead/Kite, Miltenyi Biotec, Novartis, Roche, and Takeda. R.K. is employed by Amgen; and holds stock ownership in Amgen. V.B. has received research funding from Gilead/Kite and Miltenyi Biotec; educational grants from BMS, Novartis, Takeda, and Roche; served as a consultant/advisor for Amgen, Gilead/Kite, Novartis, Pfizer, and Priothera; and serves on the speakers’ bureau for Novartis and Pfizer. S.K. has received honoraria from Cymab, Plectonic, TCR2 Inc, Miltenyi Biotec, Galapagos, Novartis, BMS, and GlaxoSmithKline; is an inventor of several patents in the field of immuno-oncology; received license fees from TCR2 Inc and Carina Biotech; and received research support from TCR2 Inc, Tabby Therapeutics, CatalYm GmbH, Plectonic GmbH, and Arcus Biosciences for work unrelated to the manuscript. The remaining authors declare no competing financial interests.
Figures

Comment in
-
STING activation enhances immunotherapy of AML.Blood. 2025 May 8;145(19):2106-2108. doi: 10.1182/blood.2025028653. Blood. 2025. PMID: 40338576 No abstract available.
References
-
- Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32(4):288–296. - PubMed
-
- Gale RP, Horowitz MM, Ash RC, et al. Identical-twin bone marrow transplants for leukemia. Ann Intern Med. 1994;120(8):646–652. - PubMed
-
- Orti G, Barba P, Fox L, Salamero O, Bosch F, Valcarcel D. Donor lymphocyte infusions in AML and MDS: enhancing the graft-versus-leukemia effect. Exp Hematol. 2017;48:1–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

