Transformation of flg22 perception into electrical signals decoded in vasculature leads to sieve tube blockage and pathogen resistance
- PMID: 40009682
- PMCID: PMC11864193
- DOI: 10.1126/sciadv.ads6417
Transformation of flg22 perception into electrical signals decoded in vasculature leads to sieve tube blockage and pathogen resistance
Abstract
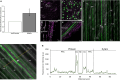
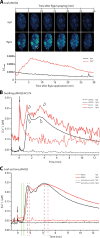
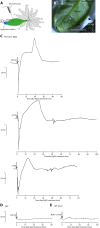
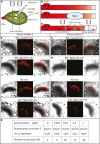
This study focuses on the question how and where information acquired by FLS2 perception of flg22 is transformed into electrical signals crucial for generation of local and systemic defense responses. In Arabidopsis thaliana and Vicia faba leaves, FLS2 density was high in the epidermis and vascular parenchyma, low in mesophyll, and absent in sieve elements (SEs). Aequorin-based examinations disclosed dual cytosolic Ca2+ peaks shortly after flg22 application, which corresponded with two voltage shifts from the epidermis to SEs. These signals were converted into rapid long-range action potentials (APs) or slower short-range variation potentials (VPs). Modified phytohormone-levels demonstrated systemic AP effects. Jasmonic acid up-regulation was significantly higher in wild-type than Atseor1/2 mutants. Abundant Ca2+ influx associated with VPs was responsible for transient sieve element occlusion (SEO) near the flg22 perception site, whereas SEO was absent in Atseor1/2 and Atfls2 mutants. Biological relevance of SEO was demonstrated by higher susceptibility of Atseor1/2 mutants to Pseudomonas syringae than wild-type plants.
Figures



Similar articles
-
Bacillus cereus AR156 Activates Defense Responses to Pseudomonas syringae pv. tomato in Arabidopsis thaliana Similarly to flg22.Mol Plant Microbe Interact. 2018 Mar;31(3):311-322. doi: 10.1094/MPMI-10-17-0240-R. Epub 2018 Jan 8. Mol Plant Microbe Interact. 2018. PMID: 29090631
-
The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling.Mol Plant Pathol. 2017 Sep;18(7):937-948. doi: 10.1111/mpp.12457. Epub 2016 Sep 15. Mol Plant Pathol. 2017. PMID: 27399963 Free PMC article.
-
The activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition.PLoS One. 2014 Feb 25;9(2):e88951. doi: 10.1371/journal.pone.0088951. eCollection 2014. PLoS One. 2014. PMID: 24586453 Free PMC article.
-
The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance.Plant Physiol. 2006 Aug;141(4):1666-75. doi: 10.1104/pp.106.081257. Epub 2006 Jun 15. Plant Physiol. 2006. PMID: 16778014 Free PMC article.
-
Allelic variation in two distinct Pseudomonas syringae flagellin epitopes modulates the strength of plant immune responses but not bacterial motility.New Phytol. 2013 Nov;200(3):847-860. doi: 10.1111/nph.12408. Epub 2013 Jul 19. New Phytol. 2013. PMID: 23865782 Free PMC article.
Cited by
-
Epidermal electronic-tattoo for plant immune response monitoring.Nat Commun. 2025 Apr 4;16(1):3244. doi: 10.1038/s41467-025-58584-x. Nat Commun. 2025. PMID: 40185801 Free PMC article.
References
-
- Boller T., Felix G., A renaissance of elicitors: Perception of microbe-associated molecular pattern and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009). - PubMed
-
- Ranf S., Eschen-Lippold L., Pecher P., Lee J., Scheel D., Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 68, 100–113 (2011). - PubMed
-
- Zhang J., Zhou J.-M., Plant immunity triggered by microbial molecular signatures. Mol. Plant 3, 783–793 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

