Modulation of blood-tumor barrier transcriptional programs improves intratumoral drug delivery and potentiates chemotherapy in GBM
- PMID: 40009687
- PMCID: PMC11864199
- DOI: 10.1126/sciadv.adr1481
Modulation of blood-tumor barrier transcriptional programs improves intratumoral drug delivery and potentiates chemotherapy in GBM
Abstract
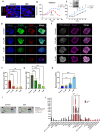
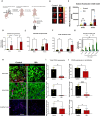
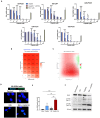
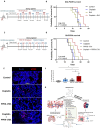
Efficient drug delivery to glioblastoma (GBM) is a major obstacle as the blood-brain barrier (BBB) and the blood-tumor barrier (BTB) prevent passage of the majority of chemotherapies into the brain. Here, we identified a transcriptional 12-gene signature associated with the BTB in GBM. We identified CDH5 as a core molecule in this set and confirmed its expression in GBM vasculature using transcriptomics and immunostaining of patient specimens. The indirubin-derivative, 6-bromoindirubin acetoxime (BIA), down-regulates CDH5 and other BTB signature genes, causing endothelial barrier disruption in vitro and in murine GBM xenograft models. Treatment with BIA increased intratumoral cisplatin accumulation and potentiated DNA damage by targeting DNA repair pathways. Last, using an injectable BIA nanoparticle formulation, PPRX-1701, we significantly improved cisplatin efficacy in murine GBM. Our work reveals potential targets of the BTB and the bifunctional properties of BIA as a BTB modulator and a potentiator of chemotherapy, supporting its further development.
Figures



Update of
-
Modulation of blood-tumor barrier transcriptional programs improves intra-tumoral drug delivery and potentiates chemotherapy in GBM.bioRxiv [Preprint]. 2024 Aug 28:2024.08.26.609797. doi: 10.1101/2024.08.26.609797. bioRxiv. 2024. Update in: Sci Adv. 2025 Feb 28;11(9):eadr1481. doi: 10.1126/sciadv.adr1481. PMID: 39253453 Free PMC article. Updated. Preprint.
References
-
- Steeg P. S., The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 18, 696–714 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

