Dysregulated balance of D- and L-amino acids modulating glutamatergic neurotransmission in severe spinal muscular atrophy
- PMID: 40010612
- PMCID: PMC11980034
- DOI: 10.1016/j.nbd.2025.106849
Dysregulated balance of D- and L-amino acids modulating glutamatergic neurotransmission in severe spinal muscular atrophy
Abstract
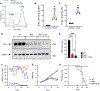
Spinal muscular atrophy (SMA) is a neuromuscular disorder caused by reduced expression of the survival motor neuron (SMN) protein. In addition to motor neuron survival, SMN deficiency affects the integrity and function of afferent synapses that provide glutamatergic excitatory drive essential for motor neuron firing and muscle contraction. However, it is unknown whether deficits in the metabolism of excitatory amino acids and their precursors contribute to neuronal dysfunction in SMA. To address this issue, we measured the levels of the main neuroactive D- and L-amino acids acting on glutamatergic receptors in the central nervous system of SMN∆7 mice as well as the cerebrospinal fluid (CSF) of SMA patients of varying severity before and after treatment with the SMN-inducing drug Nusinersen. Our findings reveal that SMN deficiency is associated with disruption of glutamate and serine metabolism in the CSF of severe SMA patients, including decreased concentration of L-glutamate, which is partially corrected by Nusinersen therapy. Moreover, we identify dysregulated l-glutamine/L-glutamate ratio as a shared neurochemical signature of altered glutamatergic synapse metabolism that implicates neuron-astrocyte dysfunction in both severe SMA patients and mouse models. Lastly, consistent with hypo-glutamatergic neurotransmission in SMA, we show that daily supplementation with the NMDA receptor co-agonist d-serine improves neurological deficits in SMN∆7 mice. Altogether, these findings provide direct evidence for central dysregulation of D- and L-amino acid metabolism linked to glutamatergic neurotransmission in severe SMA and have potential implications for treating this neurological disorder.
Keywords: Central nervous system; Cerebrospinal fluid; Glutamatergic neurotransmission; NMDA receptors; Nusinersen; Spinal muscular atrophy; d-serine.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest C.B. received advisory board honoraria from Avexis, Biogen, Novartis and Roche. The other authors declare no competing interests.
Figures


Update of
-
Dysregulated balance of D- and L-amino acids modulating glutamatergic neurotransmission in severe spinal muscular atrophy.bioRxiv [Preprint]. 2024 Oct 22:2024.10.22.619645. doi: 10.1101/2024.10.22.619645. bioRxiv. 2024. Update in: Neurobiol Dis. 2025 Apr;207:106849. doi: 10.1016/j.nbd.2025.106849. PMID: 39484528 Free PMC article. Updated. Preprint.
References
-
- Abati E, et al., 2020. Glial cells involvement in spinal muscular atrophy: could SMA be a neuroinflammatory disease?, 140, 104870. - PubMed
-
- Andersen JV, et al., 2021. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 196, 108719. - PubMed
-
- Bough KJ, et al., 2007. Evidence against enhanced glutamate transport in the anticonvulsant mechanism of the ketogenic diet. Epilepsy Res. 74, 232–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

