Effector T cells under hypoxia have an altered transcriptome similar to tumor-stressed T cells found in non-responsive melanoma patients
- PMID: 40010774
- PMCID: PMC12086921
- DOI: 10.1136/jitc-2024-010153
Effector T cells under hypoxia have an altered transcriptome similar to tumor-stressed T cells found in non-responsive melanoma patients
Abstract
Background: In the tumor microenvironment (TME), hypoxia stands as a significant factor that modulates immune responses, especially those driven by T cells. As T cell-based therapies often fail to work in solid tumors, this study aims to investigate the effects of hypoxia on T cell topo-distribution in the TME, gene expression association with T cell states, and clinical responses in melanoma.
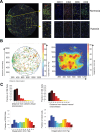
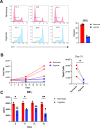
Methods: To generate detailed information on tumor oxygenation and T cell accessibility, we used mathematical modeling of human melanoma tissue microarrays that incorporate oxygen supply from vessels, intratumoral diffusion, and cellular uptake. We created tumor maps and derived plots showing the fraction of CD4 and CD8 T cells against the distance to the nearest vessel and oxygen pressure. To assess their function and transcriptional changes caused by hypoxia, effector T cells were generated and cultured under hypoxia (0.5% oxygen) or normoxia (21% oxygen). The T cell hypoxia-transcriptional signature was compared against datasets from msigDB, iATLAS (clinical trials of melanoma patients treated with immune checkpoint inhibitors (ICIs)), ORIEN AVATAR (real-world melanoma patients treated with ICIs), and a single-cell atlas of tumor-infiltrating lymphocytes.
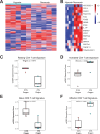
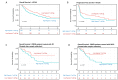
Results: We made three specific observations: (1) in melanoma T cells preferentially accumulated in oxygenated areas close to blood vessels (50-100 µm from the vasculature in the regions of high oxygen availability) but not in hypoxic areas far from blood vessels. (2) Our analysis confirmed that under hypoxia, T cell functions were significantly reduced compared with normoxic conditions and accompanied by a unique gene signature. Furthermore, this hypoxic gene signature was prevalent in resting and non-activated T cells. Notably and clinically relevant, the hypoxic T cell gene set was found to correlate with reduced overall survival and reduced progression-free survival in melanoma patients, which was more pronounced in non-responder patients undergoing ICI therapy. (3) Finally, compared with a single-cell atlas of tumor-infiltrating T cells, our hypoxia signature aligned with a population of cells at a state termed stress response state (TSTR).
Conclusions: Our study highlights the critical role of hypoxia in shaping T cell distribution and its correlation with clinical outcomes in melanoma. We revealed a preferential accumulation of T cells in oxygenated areas. Moreover, hypoxic T cells develop a distinct hypoxic gene signature prevalent in resting, non-activated T cells and TSTR that was also associated with poorer outcomes, particularly pronounced among non-responders to ICIs.
Keywords: Gene expression profiling - GEP; Immune Checkpoint Inhibitor; Melanoma; Tumor infiltrating lymphocyte - TIL; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
