Development of live biotherapeutic products: a position statement of Asia-Pacific Microbiota Consortium
- PMID: 40011030
- PMCID: PMC12013581
- DOI: 10.1136/gutjnl-2024-334501
Development of live biotherapeutic products: a position statement of Asia-Pacific Microbiota Consortium
Abstract
Objective: Live biotherapeutic products (LBPs) are biological products composed of living micro-organisms, developed to prevent, treat, or cure diseases. Examples include cultured strains of Akkermansia muciniphila and Christensenella minuta, as well as treatments using purified Firmicutes spores for recurrent Clostridioides difficile infections. There is a need for guidelines over the increasing interest in developing LBPs. A panel of microbiome experts from Asia-Pacific countries articulates their perspectives on key considerations for LBP development.
Design: Experts in microbiome research, microbiology, gastroenterology, internal medicine and biotherapeutics industry were invited to form a panel. During the 2023 Inauguration Conference of the Asia-Pacific Microbiota Consortium, an organised, iterative roundtable discussion was conducted to build expert consensus on critical issues surrounding the development of LBP.
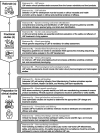
Results: The consensus statements were organised into three main aspects: (a) rationales of LBP development, (b) preclinical studies and (c) preparation for clinical studies. The panel strongly recommended to prioritise human-derived and food-sourced strains for development, with indications based on clinical need and efficacy shown in studies. Preclinical evaluation should involve thorough screening, genotyping and phenotyping, as well as comprehensive in vitro and animal studies to assess functional mechanisms and microbiological safety. Rigorous cell banking practices and genetic monitoring are essential to ensure product consistency and safety throughout the manufacturing process. Clinical trials, including postmarketing surveillance, must be carefully designed and closely monitored, with robust safety and risk management protocols in place.
Conclusions: The development of LBP should be approached with a strong emphasis on microbiological evaluation, clinical relevance, scientific mechanisms and safety at every stage. These measures are essential to ensure the safety, effectiveness and long-term success of the product.
Keywords: ENTERIC BACTERIAL MICROFLORA; MICROBIOME; PROBIOTICS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
