Broadly neutralizing antibodies isolated from HEV convalescents confer protective effects in human liver-chimeric mice
- PMID: 40011441
- PMCID: PMC11865592
- DOI: 10.1038/s41467-025-57182-1
Broadly neutralizing antibodies isolated from HEV convalescents confer protective effects in human liver-chimeric mice
Abstract
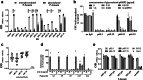
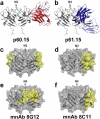
Hepatitis E virus (HEV) causes 3.3 million symptomatic cases and 44,000 deaths per year. Chronic infections can arise in immunocompromised individuals, and pregnant women may suffer from fulminant disease as a consequence of HEV infection. Despite these important implications for public health, no specific antiviral treatment has been approved to date. Here, we report combined functional, biochemical, and X-ray crystallographic studies that characterize the human antibody response in convalescent HEV patients. We identified a class of potent and broadly neutralizing human antibodies (bnAbs), targeting a quaternary epitope located at the tip of the HEV capsid protein pORF2 that contains an N-glycosylation motif and is conserved across members of the Hepeviridae. These glycan-sensitive bnAbs specifically recognize the non-glycosylated pORF2 present in infectious particles but not the secreted glycosylated form acting as antibody decoy. Our most potent bnAb protects human liver-chimeric mice from intraperitoneal HEV challenge and co-housing exposure. These results provide insights into the bnAb response to this important emerging pathogen and support the development of glycan-sensitive antibodies to combat HEV infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.S., K.D., T.K., and P.B. are listed as inventors on a patent “BROADLY NEUTRALIZING ANTIBODIES AGAINST HEPATITIS E VIRUS” describing the use of glycan-sensitive antibodies p60.1 and p60.12 targeting HEV pORF2 for diagnostics, prevention and treatment of HEV infection (EP 22 162 453.9). These authors declare no restrictions on the publication of data and the remaining authors declare no competing interests.
Figures



References
-
- Van der Poel, W. H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol.4, 91–96 (2014). - PubMed
-
- Kamar, N. et al. Hepatitis E virus infection. Nat. Rev. Dis. Prim.3, 17086 (2017). - PubMed
-
- Ingiliz, P. et al. Persisting hepatitis E virus infection leading to liver cirrhosis despite recovery of the immune system in an HIV-infected patient. Clin. Res Hepatol. Gastroenterol.40, e23–e25 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- TI 07.001_005/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- 9A888/Volkswagen Foundation (VolkswagenStiftung)
- 390874280/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 439226007/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 405772731/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources

