Microbiome modulation of implant-related infection by a novel miniaturized pulsed electromagnetic field device
- PMID: 40011461
- PMCID: PMC11865433
- DOI: 10.1038/s41522-025-00667-0
Microbiome modulation of implant-related infection by a novel miniaturized pulsed electromagnetic field device
Abstract
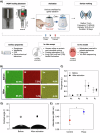
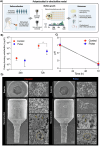
Dental implant-related infections, which lack effective therapeutic strategies, are considered the primary cause for treatment failure. Pulsed electromagnetic field (PEMF) technology has been introduced as a safe and effective modality for enhancing biological responses. However, the PEMF effect on modulating microbial diversity has not been explored. Thus, we tested a miniaturized PEMF biomedical device as a healing component for dental implants. PEMF activation did not alter the chemical composition, surface roughness, wettability, and electrochemical performance. PEMF effectively controlled chronic in vitro polymicrobial microbial accumulation. The in vivo study where devices were inserted in the patients' oral cavities and 16S RNA sequencing analysis evidenced a fivefold or more reduction in 23 bacterial species for PEMF group and the absence of some species for this group, including pathogens associated with implant-related infections. PEMF altered bacterial interactions and promoted specific bacterial pathways. PEMF has emerged as an effective strategy for controlling implant-related infections.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Shlomo Barak work at Magdent Company. The other authors declare no competing interested.
Figures






References
-
- Lybrand, K. E. & Althausen, P. L. The role of value-based implants in orthopedic trauma. Orthop. Clin. North Am.49, 437–443 (2018). - PubMed
-
- Pedrinaci, I. et al. Implant survival in the anterior mandible: a retrospective cohort study. Clin. Oral. Implants Res.34, 463–474 (2023). - PubMed
-
- Pjetursson, B. E. et al. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral. Implants Res.18, 97–113 (2007). - PubMed
-
- Albrektsson, T. & Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res.21, 4–7 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

