Mycoplasma pneumoniae MLST detected in the upsurge of pneumonia during the 2023 to 2024 winter season in the Netherlands
- PMID: 40011487
- PMCID: PMC11865550
- DOI: 10.1038/s41598-025-88990-6
Mycoplasma pneumoniae MLST detected in the upsurge of pneumonia during the 2023 to 2024 winter season in the Netherlands
Abstract
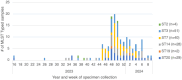
During the winter 2023-2024, an upsurge of Mycoplasma pneumoniae (M.pneumoniae) was noted in the Netherlands. To investigate the distribution of M.pneumoniae sequence types from different patient populations and to explore genotypic macrolide resistance which is common in East Asia but not (yet) in Europe. M.pneumoniae positive throat/nasal samples from participatory respiratory surveillance, patients visiting general practitioners with an acute respiratory infection including community acquired pneumonia (CAP) and hospitalised patients with CAP were included, representing different disease severity. The M.pneumoniae were typed with multilocus sequence typing and the 23 S rRNA region was sequenced to determine macrolide resistance markers. In total, 153 M.pneumoniae were sequenced, six sequence types (STs) and only one bacterium with macrolide resistance marker were detected. No link between STs or bacterial load (PCR cycle threshold) and source population of M.pneumoniae was detected. In the Netherlands, the M.pneumoniae upsurge in 2023-2024 existed of multiple commonly found STs. No link between ST and severity of illness was detected. Macrolide resistance remained sporadic.
Keywords: Mycoplasma pneumoniae; CAP; Community; General practitioner; Hospital; MLST types; Other acute respiratory illness; Resistance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: Non-WMO approval (WMO = Wet Medisch-wetenschappelijk Onderzoek met mensen; the Dutch law regulating medical scientific research with humans) was obtained at Maasstad hospital (L2024027) and Franciscus Gasthuis and Vlietland, Rotterdam the Netherlands (2024-031). For research based on data and samples collected in the GP sentinel surveillance a waiver for ethical approval was obtained from the Clinical Expert Centre RIVM (IDS-688). For Infectieradar a waiver for ethical approval was obtained from the Medical Ethics Review Committee Utrecht (reference number: WAG/avd/20/008757; protocol 20–131) given the nature of data collection. Due to the retrospective nature of the study, the Medical Ethics Review Committee Utrecht, the institutional review boards at Expert Centre RIVM, Maasstad hospital, and the institutional review board at Franciscus Gasthuis and Vlietland waived the need of obtaining informed consent. Hereby authors confirm that all experiments were performed in accordance with relevant guidelines and regulations that apply for this retrospective study on residual clinical samples.
Figures

References
-
- RIVM. Virologische weekstaten. https://www.rivm.nl/virologische-weekstaten.
-
- Chen, Y., Li, X., Fu, Y., Yu, Y. & Zhou, H. Whole-genome sequencing unveils the outbreak of Mycoplasma pneumoniae in mainland China. Lancet Microbe6, 66 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

