DFT insights into bandgap engineering of lead-free LiMCl3 (M = Mg, Be) halide perovskites for optoelectronic device applications
- PMID: 40011532
- PMCID: PMC11865626
- DOI: 10.1038/s41598-025-90621-z
DFT insights into bandgap engineering of lead-free LiMCl3 (M = Mg, Be) halide perovskites for optoelectronic device applications
Abstract
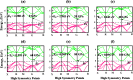
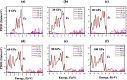
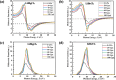
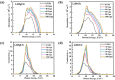
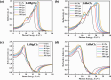
In this theoretical analysis, the pressure-dependent structural, electronic, mechanical, and optoelectronic properties of LiMCl3 (M = Mg, Be) have been calculated using density functional theory within the framework of the GGA PBE and hybrid HSE06 functional. At ambient pressure, the calculated lattice parameters of LiMCl3 match well with previously reported values, validating the accuracy of this study. Geometry optimization reveals that under increasing hydrostatic pressure, both the lattice parameters and the unit cell volume decrease. Additionally, the band structure exhibits notable phenomena over the pressure range from 0 to 100 GPa. For the LiMgCl3 compound, the bandgap decreases from an indirect bandgap of 4 eV to a direct bandgap of 2.563 eV. Similarly, LiBeCl3 shows an indirect bandgap that decreases from 2.388 eV to 0.096 eV over the pressure range from 0 to 100 GPa. The optical properties of LiMCl3, including absorption coefficient, reflectivity, refractive index, dielectric function, and conductivity, have been calculated throughout the study under varying pressure conditions. The analysis reveals that the optical properties of LiMCl3 (M = Be, Mg) enhance with increasing hydrostatic pressure, thereby rendering these materials more suitable for optoelectronic applications. To assess the stability of these compounds, elastic constants were analyzed, indicating that LiMCl3 exhibits ductile and anisotropic characteristics under different pressure conditions. These investigated materials are suitable for use in optoelectronic devices due to their favorable physical properties under different pressure circumstances.
Keywords: Bandgap engineering; DFT; Hydrostatic pressure; Lead-free perovskite; Mechanical properties; Optical properties.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures








References
-
- Pandit, N. et al. Study of chalcogenide-based metal perovskites BaZrX3 (X = S and Se): DFT insight into fundamental properties for sustainable energy generation using AMPS-1D. J. Comput. Electron.23, 1014–1028 (2024).
-
- Boutramine, A. et al. A theoretical investigation of the Ba2CePtO6 double perovskite for optoelectronic and thermoelectric applications. Opt. Quantum Electron.56, 395 (2024).
-
- Khuili, M. et al. First-principles study of halide double perovskite cs 2 SnX 6 (X = cl, br, I) for solar cell applications. Mod. Phys. Lett. B. 38, 2350223 (2024).
-
- Abbas, Z. et al. DFT insights for structural, opto-electronic, thermodynamic and transport characteristics of Tl2TeX6 (X = at, br, cl, I) double perovskites for low-cost solar cell applications. Inorg. Chem. Commun.163, 112293 (2024).
-
- Boutramine, A. et al. First-principles investigations of structural, thermodynamic, optoelectronic and thermoelectric properties of Rb2CuMF6 (M = As3+, Bi3+) eco-friendly halide double perovskites: materials for green energy applications. J. Inorg. Organomet. Polym. Mater.34, 4374–4391 (2024).
LinkOut - more resources
Full Text Sources

