Costunolide normalizes neuroinflammation and improves neurogenesis deficits in a mouse model of depression through inhibiting microglial Akt/mTOR/NF-κB pathway
- PMID: 40011631
- PMCID: PMC12205059
- DOI: 10.1038/s41401-025-01506-w
Costunolide normalizes neuroinflammation and improves neurogenesis deficits in a mouse model of depression through inhibiting microglial Akt/mTOR/NF-κB pathway
Abstract
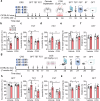
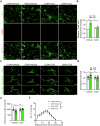
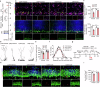
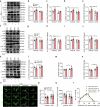
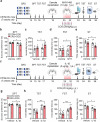
Neuroinflammation is crucial for the pathogenesis of major depression. Preclinical studies have shown the potential of anti-inflammatory agents, specifically costunolide (COS), correlate with antidepressant effects. In this study, we investigated the molecular mechanisms underlying the antidepressant actions of COS. Chronic restraint stress (CRS) was induced in male mice. The mice were treated with either intra-DG injection of COS (5 μM, 1 μL per side) or COS (20 mg/kg, i.p.) for 1 week. We showed that administration of COS through the both routes significantly ameliorated the depressive-like behavior in CRS-exposed mice. Furthermore, administration of COS significantly improved chronic stress-induced adult hippocampal neurogenesis deficits in the mice through attenuating microglia-derived neuroinflammation. We demonstrated that COS (5 μM) exerted anti-neuroinflammatory effects in LPS-treated BV2 cells via inhibiting microglial Akt/mTOR/NF-κB pathway; inactivation of mTOR/NF-κB/IL-1β pathway was required for the pro-neurogenic action of COS in CRS-exposed mice. Our results reveal the antidepressant mechanism of COS that is normalizing neuroinflammation to improve neurogenesis deficits, supporting anti-inflammatory agents as a potential therapeutic strategy for depression.
Keywords: chronic stress; costunolide; mTOR; major depression; microglia; neurogenesis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Smith K. Mental health: a world of depression. Nature. 2014;515:181. - PubMed
-
- First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. 2013;201:727–9. - PubMed
-
- Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry. 2018;5:339–47. - PubMed
-
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

