Iron trapping in macrophages reshapes the homeostasis of the haematopoietic system
- PMID: 40012014
- PMCID: PMC12078876
- DOI: 10.1111/bjh.20031
Iron trapping in macrophages reshapes the homeostasis of the haematopoietic system
Abstract
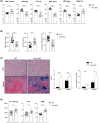
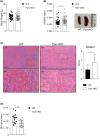
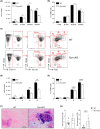
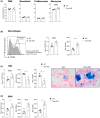
Iron is required for key physiological processes, like oxygen transport, energy production and cell proliferation. Body iron homeostasis is regulated by the erythroferrone-hepcidin-ferroportin (FPN) axis, which mainly acts on absorptive duodenal cells and macrophages involved in iron recycling from red blood cell breakdown. In addition to systemic iron regulation, macrophages are also involved in local iron release to neighbouring cells. Similarly, bone marrow (BM)-resident macrophages could represent promptly available local sources of iron for developing haematopoietic cells. To study the impact of macrophage-released iron on BM haematopoietic stem and progenitor cells, we employed mice with targeted deletion of Fpn in the myeloid lineage (Fpn conditional knockout or Fpn-cKO). Fpn-cKO mice develop age-related anaemia and microcytaemia, reduction of BM erythroblasts and preferential megakaryopoiesis at the expenses of erythropoiesis, suggesting that red cells are mostly affected by the lack of myeloid-derived iron delivery. Transferrin receptor 1 surface expression is higher in Fpn-cKO mice than littermate controls in all the BM subpopulation analysed, starting from haematopoietic stem cells, indicating a broad BM sensitivity to lower iron availability. Last, Fpn-cKO mice activate systemic compensatory mechanisms, such as extramedullary haematopoiesis and erythroferrone upregulation, albeit not sufficient to overcome anaemia.
Keywords: anaemia; animal model; haematopoiesis; iron; macrophages; red cells.
© 2025 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Recalcati S, Gammella E, Buratti P, Cairo G. Molecular regulation of cellular iron balance. IUBMB Life. 2017;69(6):389–398. - PubMed

