Radiotherapy resistance driven by Asparagine endopeptidase through ATR pathway modulation in breast cancer
- PMID: 40012043
- PMCID: PMC11866873
- DOI: 10.1186/s13046-025-03334-6
Radiotherapy resistance driven by Asparagine endopeptidase through ATR pathway modulation in breast cancer
Abstract
Background: Tumor resistance represents a major challenge in the current oncology landscape. Asparagine endopeptidase (AEP) overexpression correlates with worse prognosis and reduced overall survival in most human solid tumors. However, the underlying mechanisms of the connection between AEP and reduced overall survival in cancer patients remain unclear.
Methods: High-throughput proteomics, cellular and molecular biology approaches and clinical data from breast cancer (BC) patients were used to identify novel, biologically relevant AEP targets. Immunoblotting and qPCR analyses were used to quantify protein and mRNA levels. Flow cytometry, confocal microscopy, chemical inhibitors, siRNA- and shRNA-silencing and DNA repair assays were used as functional assays. In-silico analyses using the TCGA BC dataset and immunofluorescence assays in an independent cohort of invasive ductal (ID) BC patients were used to validate the clinical relevance of our findings.
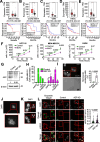
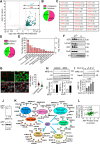
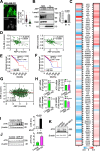
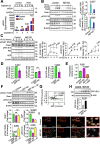
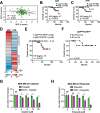
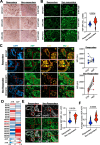
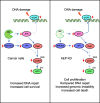
Results: Here we showed a dual role for AEP in genomic stability and radiotherapy resistance in BC patients by suppressing ATR and PPP1R10 levels. Reduced ATR and PPP1R10 levels were found in BC patients expressing high AEP levels and correlated with worst prognosis. Mechanistically, AEP suppresses ATR levels, reducing DNA damage-induced cell death, and PPP1R10 levels, promoting Chek1/P53 cell cycle checkpoint activation, allowing BC cells to efficiently repair DNA. Functional studies revealed AEP-deficiency results in genomic instability, increased DNA damage signaling, reduced Chek1/P53 activation, impaired DNA repair and cell death, with phosphatase inhibitors restoring the DNA damage response in AEP-deficient BC cells. Furthermore, AEP inhibition sensitized BC cells to the chemotherapeutic reagents cisplatin and etoposide. Immunofluorescence assays in an independent cohort of IDBC patients showed increased AEP levels in ductal cells. These analyses showed that higher AEP levels in radioresistant IDBC patients resulted in ATR nuclear eviction, revealing AEPhigh/ATRlow protein levels as an efficient predictive biomarker for the stratification of radioresistant patients.
Conclusion: The newly identified AEP/ATR/PPP1R10 axis plays a dual role in genomic stability and radiotherapy resistance in BC. Our work provides new clues to the underlying mechanisms of tumor resistance and strong evidence validating the AEP/ATR axis as a novel predictive biomarker and therapeutic target for the stratification and treatment of radioresistant BC patients.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical standards set forth in the Spanish legislation (Ley de Investigacion Organica Biomedica, 14 July 2007) and was approved by the ethical committees of the MD Anderson Cancer Centre Madrid, Spain. A comprehensive written informed consent was obtained from all participants. All authors followed the applicable ethical standards to maintain research integrity. Consent for publication: All authors have read the final version of the manuscript and agree with its publication. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
- PID2019-104195G/Spanish Ministry of Science and Innovation-Agencia Estatal de Investigación/10.13039/501100011033
- PID2022-136854OB-I00/MICINN-AEI and European Regional Development Fund
- PID2021-124251OB-I00/MICINN-AEI and European Regional Development Fund
- CB16/12/00295/The Instituto de Salud Carlos III IISCIII, CIBERONC
- AC21_2/00020/ERA PerMed ERA-NET co-funded by the NextGeneration-EU
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

