Node Modification of Metal-Organic Frameworks for Catalytic Applications
- PMID: 40012297
- PMCID: PMC12256937
- DOI: 10.1002/open.202400428
Node Modification of Metal-Organic Frameworks for Catalytic Applications
Abstract
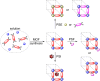
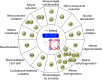
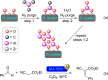
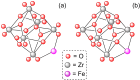
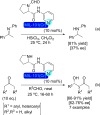
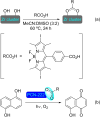
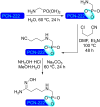
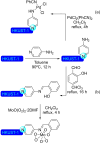
Metal-organic frameworks (MOFs) have been a breakthrough in different fields of chemistry, not only due to the extensive possibilities regarding their synthesis, but also the ease of modulation of the structure's properties by chemical modification of linkers and nodes. The latter is particularly interesting in heterogeneous catalysis, as the newly inserted species may enhance, regulate, or straight enable new forms of catalysis unattainable by the pristine material. This acts in conjunction with the parent MOFs providing selectivity (e. g., by size exclusion) and protecting highly reactive catalytic species, offering increased stability and robustness to well-known catalytic systems. In this review, we compile the most relevant post-synthetic modification of the nodes of well-known MOFs of the last decade (2015-2024) and their application to heterogeneous catalysis. This review is divided into two main sections covering modifications involving metallic species and organic moieties, with sub-sections for each MOF on both. This way, we aim to provide a broad view of the state of the art while showcasing the expanded catalytic properties of the resulting materials.
Keywords: Catalysis; Cluster; Metal-organic framework; Node; Post-synthetic modification.
© 2025 The Authors. ChemistryOpen published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





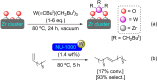

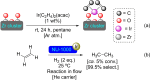
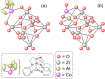
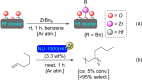

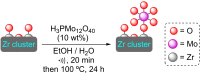
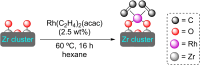


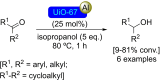
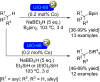
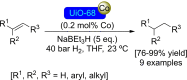

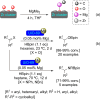




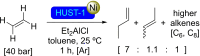

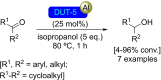


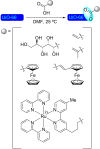


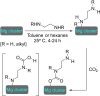
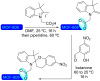
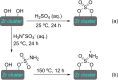
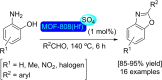

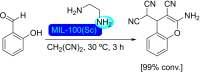
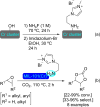
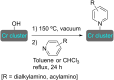

References
-
- Yaghi O. M., Li H., J. Am. Chem. Soc. 1995, 117, 10401.
-
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M., Science 2013, 341, 1230444. - PubMed
-
- Wang Q., Astruc D., Chem. Rev. 2020, 120, 1438. - PubMed
-
- Eddaoudi M., Moler D. B., Li H., Chen B., Reineke T. M., O'Keeffe M., Yaghi O. M., Acc. Chem. Res. 2001, 34, 319. - PubMed
-
- Kim J., Chen B., Reineke T. M., Li H., Eddaoudi M., Moler D. B., O'Keeffe M., Yaghi O. M., J. Am. Chem. Soc. 2001, 123, 8239. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

