Formation of polysulfides as a smart strategy to selectively detect H2S in a Bi(iii)-based MOF material
- PMID: 40012690
- PMCID: PMC11853078
- DOI: 10.1039/d4sc07144a
Formation of polysulfides as a smart strategy to selectively detect H2S in a Bi(iii)-based MOF material
Abstract
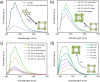
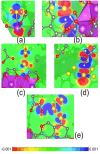
SU-101 was demonstrated to be an effective and efficient detector for H2S, due to the facile generation of polysulfides, with a remarkable H2S selectivity. Raman and XPS analyses confirmed the formation of S n 2- and S4 2- polysulfide species after the H2S adsorption (at 0.05 bar, 0.1 bar and 1 bar), without compromising the structural integrity of SU-101. The detection mechanism involves rigidification of the structure by the formation of the polysulfides and blockage of the ligand-metal charge transfer (LMCT) process, which increased the radiative emission. Additionally, theoretical simulations were carried out in order to demonstrate that the interaction of the polysulfide molecules inside the pores of SU-101 is energetically stable. Remarkably, the limit of H2S detection (LOD) was calculated to be as low as approximately 22 ppm. Finally, SU-101 is nominated as a promising candidate for implementing toxic waste valorisation (i.e., capture of toxic H2S) toward relevant applications in accurate molecular sensing.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Truong D. H. Eghbal M. A. Hindmarsh W. Roth S. H. O'Brien P. J. Drug Metab. Rev. 2006;38:733–744. - PubMed
-
- Beauchamp R. O. Bus J. S. Popp J. A. Boreiko C. J. Andjelkovich D. A. Leber P. CRC Crit. Rev. Toxicol. 1984;13:25–97. - PubMed
-
- Zeng J. Li M. Liu A. Feng F. Zeng T. Duan W. Li M. Gong M. Wen C. Yin Y. Adv. Funct. Mater. 2018;28:1800515.
-
- Meng Z. Stolz R. M. Mirica K. A. J. Am. Chem. Soc. 2019;141:11929–11937. - PubMed
LinkOut - more resources
Full Text Sources

