Immunologic and inflammatory consequences of SARS-CoV-2 infection and its implications in renal disease
- PMID: 40012912
- PMCID: PMC11861071
- DOI: 10.3389/fimmu.2024.1376654
Immunologic and inflammatory consequences of SARS-CoV-2 infection and its implications in renal disease
Abstract
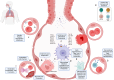
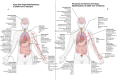
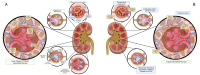
The emergence of the COVID-19 pandemic made it critical to understand the immune and inflammatory responses to the SARS-CoV-2 virus. It became increasingly recognized that the immune response was a key mediator of illness severity and that its mechanisms needed to be better understood. Early infection of both tissue and immune cells, such as macrophages, leading to pyroptosis-mediated inflammasome production in an organ system critical for systemic oxygenation likely plays a central role in the morbidity wrought by SARS-CoV-2. Delayed transcription of Type I and Type III interferons by SARS-CoV-2 may lead to early disinhibition of viral replication. Cytokines such as interleukin-1 (IL-1), IL-6, IL-12, and tumor necrosis factor α (TNFα), some of which may be produced through mechanisms involving nuclear factor kappa B (NF-κB), likely contribute to the hyperinflammatory state in patients with severe COVID-19. Lymphopenia, more apparent among natural killer (NK) cells, CD8+ T-cells, and B-cells, can contribute to disease severity and may reflect direct cytopathic effects of SARS-CoV-2 or end-organ sequestration. Direct infection and immune activation of endothelial cells by SARS-CoV-2 may be a critical mechanism through which end-organ systems are impacted. In this context, endovascular neutrophil extracellular trap (NET) formation and microthrombi development can be seen in the lungs and other critical organs throughout the body, such as the heart, gut, and brain. The kidney may be among the most impacted extrapulmonary organ by SARS-CoV-2 infection owing to a high concentration of ACE2 and exposure to systemic SARS-CoV-2. In the kidney, acute tubular injury, early myofibroblast activation, and collapsing glomerulopathy in select populations likely account for COVID-19-related AKI and CKD development. The development of COVID-19-associated nephropathy (COVAN), in particular, may be mediated through IL-6 and signal transducer and activator of transcription 3 (STAT3) signaling, suggesting a direct connection between the COVID-19-related immune response and the development of chronic disease. Chronic manifestations of COVID-19 also include systemic conditions like Multisystem Inflammatory Syndrome in Children (MIS-C) and Adults (MIS-A) and post-acute sequelae of COVID-19 (PASC), which may reflect a spectrum of clinical presentations of persistent immune dysregulation. The lessons learned and those undergoing continued study likely have broad implications for understanding viral infections' immunologic and inflammatory consequences beyond coronaviruses.
Keywords: AKI; COVID-19; PASC; SARS-CoV-2; inflammasome; inflammation; long COVID.
Copyright © 2025 Naiditch, Betts, Larman, Levi and Rosenberg.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
Figures

Similar articles
-
SARS-CoV-2 ORF3a induces COVID-19-associated kidney injury through HMGB1-mediated cytokine production.mBio. 2024 Nov 13;15(11):e0230824. doi: 10.1128/mbio.02308-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39345136 Free PMC article.
-
SARS-Cov2 acute and post-active infection in the context of autoimmune and chronic inflammatory diseases.J Transl Autoimmun. 2022;5:100154. doi: 10.1016/j.jtauto.2022.100154. Epub 2022 Apr 12. J Transl Autoimmun. 2022. PMID: 35434592 Free PMC article.
-
Inflammatory and Autoimmune Aspects of Multisystem Inflammatory Syndrome in Children (MIS-C): A Prospective Cohort Study.Viruses. 2024 Jun 12;16(6):950. doi: 10.3390/v16060950. Viruses. 2024. PMID: 38932242 Free PMC article.
-
The pathogenesis of coronavirus-19 disease.J Biomed Sci. 2022 Oct 26;29(1):87. doi: 10.1186/s12929-022-00872-5. J Biomed Sci. 2022. PMID: 36289507 Free PMC article. Review.
-
Dysregulated Interferon Response and Immune Hyperactivation in Severe COVID-19: Targeting STATs as a Novel Therapeutic Strategy.Front Immunol. 2022 May 17;13:888897. doi: 10.3389/fimmu.2022.888897. eCollection 2022. Front Immunol. 2022. PMID: 35663932 Free PMC article. Review.
Cited by
-
Metabolic Reprogramming in Respiratory Viral Infections: A Focus on SARS-CoV-2, Influenza, and Respiratory Syncytial Virus.Biomolecules. 2025 Jul 16;15(7):1027. doi: 10.3390/biom15071027. Biomolecules. 2025. PMID: 40723899 Free PMC article. Review.
-
The Role of Viral Infections in Acute Kidney Injury and Mesenchymal Stem Cell-Based Therapy.Stem Cell Rev Rep. 2025 Jun;21(5):1199-1236. doi: 10.1007/s12015-025-10873-0. Epub 2025 Apr 8. Stem Cell Rev Rep. 2025. PMID: 40198477 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

