Infant Gut Microbiota and Childhood Blood Pressure: Prospective Associations and the Modifying Role of Breastfeeding
- PMID: 40013588
- PMCID: PMC12132632
- DOI: 10.1161/JAHA.124.037447
Infant Gut Microbiota and Childhood Blood Pressure: Prospective Associations and the Modifying Role of Breastfeeding
Abstract
Background: Germ-free mice experiments indicate that human gut microbiota influence blood pressure (BP), but no studies have prospectively examined if infant gut microbiota affects their future childhood BP. We aim to investigate prospective associations of infant gut microbiota diversity and composition with childhood BP, examining effect measure modification by breastfeeding and mediation by a child's body mass index.
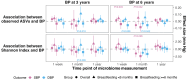
Methods and results: In the Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort, we measured infant gut microbiota (16S rRNA V4) at 1 week, 1 month, and 1 year and child BP at 3 and 6 years. We assessed α diversity-BP, β diversity-BP, and microbe abundances-BP associations using linear regression, permutational multivariate analysis of variance, and beta-binomial count regression, respectively. Data from 526 children showed that α diversity and several Bifidobacterium spp. had protective associations with BP but only in children breastfed for ≥6 months. For instance, a 1-unit increment in 1 month Shannon index was associated with 1.86 mm Hg (95% CI, 0.66-3.05) lower 6-year systolic BP in children breastfed ≥6 months but a 0.73 (95% CI, -1.00 to 2.45) higher 6-year systolic BP in those breastfed <6 months (P-interaction=0.02). Greater abundance of 2 Bifidobacterium microbes at 1 week was negatively associated with 6-year systolic BP when breastfeeding ≥6 months (P-interaction<0.1). Further, abundance of 8 microbes at 1week or 1 month was linked to 3-year or 6-year BP (false discovery rate P<0.05), with 5 of them independent of a child's body mass index. Lastly, 1-week unweighted UniFrac distance and 1-year weighted UniFrac distance were associated with BP after adjustment (P<0.05).
Conclusions: Gut microbiota features at 1 week and 1 month of life were associated with BP at 6 years. Breastfeeding duration modified key associations including those for α diversity and Bifidobacteria.
Keywords: Bifidobacteria; breastfeeding; childhood blood pressure; early‐life gut microbiota.
Conflict of interest statement
None.
Figures



References
-
- Juhola J, Oikonen M, Magnussen CG, Mikkila V, Siitonen N, Jokinen E, Laitinen T, Wurtz P, Gidding SS, Taittonen L, et al. Childhood physical, environmental, and genetic predictors of adult hypertension: the cardiovascular risk in young Finns study. Circulation. 2012;126:402–409. doi: 10.1161/CIRCULATIONAHA.111.085977 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

