Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS
- PMID: 40013621
- PMCID: PMC11906131
- DOI: 10.1530/JOE-24-0269
Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS
Abstract
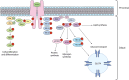
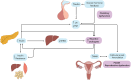
Polycystic ovary syndrome (PCOS), a reproductive endocrine disorder with quintessential features of metabolic dysfunction, affects millions of women worldwide. Hyperinsulinemia (i.e., elevated insulin without hypoglycemia) is a common metabolic feature of PCOS that worsens its reproductive symptoms by exacerbating pituitary hormone imbalances and increasing levels of bioactive androgens. Hyperinsulinemia in PCOS is often attributed to insulin resistance, based on the concept that impaired insulin-mediated glucose disposal would induce compensatory insulin hypersecretion. However, it is challenging to define the sequential relationship between insulin sensitivity and insulin secretion, as they are tightly interlinked, and evidence suggests that hyperinsulinemia can alternatively precede insulin resistance. Notably, other drivers of hyperinsulinemia (outside of insulin resistance) may be highly relevant in the context of PCOS. For instance, high androgen levels can augment both hyperinsulinemia and insulin resistance, generating a self-perpetuating cycle of reproductive and metabolic dysfunction. In this review, we evaluate the cause-and-effect relationships between insulin resistance and hyperinsulinemia in PCOS. We examine evidence for the prevailing theory of insulin resistance as the primary defect that causes secondary compensatory hyperinsulinemia, and an alternative framework of hyperinsulinemia as the earlier defect that perpetuates reproductive and metabolic features of PCOS. Considering the heterogeneous nature of PCOS, it is improbable that its metabolic characteristics always follow the same progression. Comprehensively examining all mechanistic regulators of hyperinsulinemia and insulin resistance in PCOS might thereby lead to improved prevention and management strategies, and address critical knowledge gaps in the progression of PCOS pathogenesis.
Keywords: androgens; insulin; insulin clearance; insulin hypersecretion; insulin sensitivity; metabolic dysfunction; polycystic ovary syndrome.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

