Effect of administration and withdrawal of the sodium-glucose cotransporter 2 inhibitor, tofogliflozin, on renal protection in individuals with type 2 diabetes mellitus and diabetic nephropathy: A multicenter, single-arm study (RESTORE-nephropathy study)
- PMID: 40013715
- PMCID: PMC12057378
- DOI: 10.1111/jdi.70018
Effect of administration and withdrawal of the sodium-glucose cotransporter 2 inhibitor, tofogliflozin, on renal protection in individuals with type 2 diabetes mellitus and diabetic nephropathy: A multicenter, single-arm study (RESTORE-nephropathy study)
Abstract
Aims/introduction: The mechanisms of the renoprotective effects of sodium-glucose cotransporter 2 inhibitors are unknown. This study aimed to explore the effect and mechanism of tofogliflozin on urinary albumin by administration, withdrawal, and re-administration.
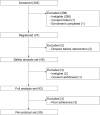
Materials and methods: Individuals with type 2 diabetes mellitus and stage 2 or 3 diabetic nephropathy were enrolled. Tofogliflozin was administered for 24 weeks, withdrawn for 12 weeks (withdrawal period), and re-administered for 24 weeks. The primary endpoint was the change in urinary albumin/creatinine ratio (UACR). The secondary endpoints included hemoglobin A1c (HbA1c), hepatic biomarkers, lipid profiles, physical examinations, and blood counts.
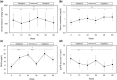
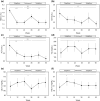
Results: A total of 47 individuals were enrolled. UACR significantly decreased throughout the observation period. It also significantly decreased, increased, and again decreased during the period of the 1st administration, withdrawal, and re-administration, respectively. HbA1c, body weight, waist circumference, and systolic blood pressure also showed the same tendency. Aspartate aminotransferase and alanine aminotransferase significantly decreased throughout the observation period, but did not increase during the withdrawal period.
Conclusions: Urinary albumin improved during the administration of tofogliflozin and worsened during its withdrawal, suggesting the reversibility of its renoprotective effect. The administration of tofogliflozin should be continued to avoid the reversal of glycemic control, renoprotective effects, and other beneficial effects.
Keywords: Diabetic nephropathy; Drug withdrawal; Renal protection; Tofogliflozin; Urinary albumin.
© 2025 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures

References
-
- Hanafusa N, Abe M, Joki N, et al. 2022 annual dialysis data report, JSDT renal data registry. J Jpn Soc Dial Ther 2023; 56: 473–536.
-
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. - PubMed
-
- Gaede P, Lund‐Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. - PubMed
-
- Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

