Host Plasma Microenvironment in Immunometabolically Impaired HIV Infection Leads to Dysregulated Monocyte Function and Synaptic Transmission Ex Vivo
- PMID: 40013867
- PMCID: PMC12021100
- DOI: 10.1002/advs.202416453
Host Plasma Microenvironment in Immunometabolically Impaired HIV Infection Leads to Dysregulated Monocyte Function and Synaptic Transmission Ex Vivo
Abstract
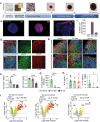
Risk stratification using multi-omics data deepens understanding of immunometabolism in successfully treated people with HIV (PWH) is inadequately explained. A personalized medicine approach integrating blood cell transcriptomics, plasma proteomics, and metabolomics is employed to identify the mechanisms of immunometabolic complications in prolonged treated PWH from the COCOMO cohort. Among the PWHs, 44% of PWH are at risk of experiencing immunometabolic complications identified using the network-based patient stratification method. Utilizing advanced machine learning techniques and a Bayesian classifier, five plasma protein biomarkers; Tubulin Folding Cofactor B (TBCB), Gamma-Glutamylcyclotransferase (GGCT), Taxilin Alpha (TXLNA), Pyridoxal Phosphate Binding Protein (PLPBP) and Large Tumor Suppressor Kinase 1 (LATS1) are identified as highly differentially abundant between healthy control (HC)-like and immunometabolically at-risk PWHs (all FDR<10-10). The personalized metabolic models predict metabolic perturbations, revealing disruptions in central carbon metabolic fluxes and host tryptophan metabolism in at-risk phenotype. Functional assays in primary cells and cortical forebrain organoids (FBOs) further validate this. Metabolic perturbations lead to persistent monocyte activation, thereby impairing their functions ex vivo. Furthermore, the chronic inflammatory plasma microenvironment contributes to synaptic dysregulation in FBOs. The endogenous plasma inflammatory microenvironment is responsible for chronic inflammation in treated immunometabolically complicated at-risk PWH who have a higher risk of cardiovascular and neuropsychiatric disorders.
Keywords: HIV/AIDS; Integrative omics; patient stratification; personalized metabolic models.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
O'Mahony has served on speaker bureaus for Abbott, Nestlé, Nutricia, Reckitt and Yakult; received research funding from Chiesi and GSK; and acted as a consultant for PrecisionBiotics. MR is an Olink Proteomics, Boston, Massachusetts, United States employee. VP was an employee of Olink GmbH, Munich, DE, when the study was conducted and is presently Novo Nordisk, Denmark employee. RB was an employee at NBIS at the time of data analyses. RB is currently an employee of Chiesi Farmaceutici S.p.A. and does not hold Chiesi Farmaceutici S.p.A. stocks or equity shares. Chiesi Farmaceutici S.p.A. was not involved with the current study. Others none to declare.
Figures





References
-
- a) Brunet‐Ratnasingham E., Dube M., Kaufmann D. E., Trends Mol. Med. 2019, 25, 1; - PubMed
- b) Ambikan A. T., Svensson‐Akusjärvi S., Krishnan S., Sperk M., Nowak P., Vesterbacka J., Sönnerborg A., Benfeitas R., Neogi U., Life Sci. Allia. 2022, 5, e202201405; - PMC - PubMed
- c) Mikaeloff F., Svensson Akusjarvi S., Ikomey G. M., Krishnan S., Sperk M., Gupta S., Magdaleno G. D. V., Escos A., Lyonga E., Okomo M. C., Tagne C. T., Babu H., Lorson C. L., Vegvari A., Banerjea A. C., Kele J., Hanna L. E., Singh K., de Magalhaes J. P., Benfeitas R., Neogi U., Commun. Biol. 2022, 5, 27. - PMC - PubMed
-
- a) Akusjarvi S. S., Ambikan A. T., Krishnan S., Gupta S., Sperk M., Vegvari A., Mikaeloff F., Healy K., Vesterbacka J., Nowak P., Sonnerborg A., Neogi U., iScience 2022, 25, 103607; - PMC - PubMed
- b) Salguero S., Brochado‐Kith O., Verdices A. V., Berenguer J., Gonzalez‐Garcia J., Martinez I., Diez C., Hontanon V., Perez‐Latorre L., Fernandez‐Rodriguez A., Jimenez‐Sousa M. A., Resino S., Biomed. Pharmacother.=Biomed. Pharmacother. 2023, 159, 114220; - PubMed
- c) Wedrychowski A., Martin H. A., Li Y., Telwatte S., Kadiyala G. N., Melberg M., Etemad B., Connick E., Jacobson J. M., Margolis D. M., Skiest D., Volberding P., Hecht F., Deeks S., Wong J. K., Li J. Z., Yukl S. A., J. Virol. 2023, 97, e0125422; - PMC - PubMed
- d) Wang S., Zhang Q., Hui H., Agrawal K., Karris M. A. Y., Rana T. M., Emerg. Microb. Infect. 2020, 9, 2333. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
