Efficacy and tolerability of low versus standard daily doses of antiseizure medications in newly diagnosed focal epilepsy. A multicenter, randomized, single-blind, non-inferiority trial (STANDLOW)
- PMID: 40013886
- PMCID: PMC12014934
- DOI: 10.1002/epi4.70016
Efficacy and tolerability of low versus standard daily doses of antiseizure medications in newly diagnosed focal epilepsy. A multicenter, randomized, single-blind, non-inferiority trial (STANDLOW)
Abstract
Objective: The STANDLOW trial investigated whether first-line antiseizure monotherapy with low doses has a similar efficacy to standard doses, but with fewer adverse events, improved quality of life, and reduced costs for the National Health System.
Methods: Multicenter, randomized, parallel-arm, single-blind, non-inferiority trial, comparing low dose versus standard dose of antiseizure medications (carbamazepine, levetiracetam, valproate, zonisamide, oxcarbazepine, topiramate, lamotrigine, gabapentin, lacosamide) in adults with newly diagnosed focal epilepsy.
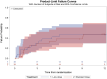
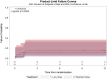
Results: The intention-to-treat (ITT) population consisted of 58 randomized patients, 29 in the low dose arm and 29 in the standard dose arm, 27 (46.6%) females and 31 (53.4%) males, with an age between 18 and 87 years (median 54.9, IQR 32-71). The seizure type was focal impaired awareness seizures in 44 (75.9%) and focal aware seizures in 14 (24.1%). Etiology was unknown in 43 (74.1%) and structural in 15 (25.9%). At study entry, EEG was epileptiform in 28 (48.2%) and seizure frequency was low (≤2 seizures/month) in 41 (70.7%). The estimated relapse proportions at 12 months were 47% for the low dose and 48% for the standard dose, with a difference of 1% (95% CI: -30%; 27%). At the end of the study visit (12 months of follow-up, or immediately after seizure relapse or study withdrawal for other reasons, whichever came first), no differences in the number or severity of adverse events or quality of life measures were observed between the two treatment groups. The total drug-related costs over the entire study period were lower in the low dose arm (median per participant 253 € versus 475 € in the standard dose arm).
Significance: Although the efficacy of low doses versus standard doses appeared similar, non-inferiority could not be demonstrated due to slow recruitment and premature termination of the trial. Although statistically inconclusive, our findings suggest that a low dose of antiseizure medications may be considered as a first-line option in adult patients with a new diagnosis of focal epilepsy of unknown etiology and low seizure frequency.
Plain language summary: This study aimed to see if low doses of anti-seizure medications (ASMs) could be as effective as standard doses in treating adults with newly diagnosed epilepsy. Subjects were assigned to receive either a low or standard dose of ASMs. 58 adults participated. Both low and standard doses seemed to have a similar effect on controlling seizures. The study was stopped early due to slow enrollment, making it difficult to definitively prove that low doses were non-inferior to standard doses. Low doses of ASMs might be a reasonable option for adults with newly diagnosed epilepsy with no clear cause and few seizures.
Keywords: efficacy; focal epilepsy; low dose; monotherapy; newly‐diagnosed epilepsy; randomized trial.
© 2025 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
E. Bianchi, G. Giussani, S. Beretta, J.C. Di Francesco, M. Cecconi, R. Papetti, E. Rosati, G. Giovannelli, D. Benincasa, M. Longoni, Y. Bartolini, A. Alicino, E. Pronello, V. Cianci, P. Tabaee Damavandi, S. Filipponi, A. Gaiani, S. Diamanti, F. Pasini, G. Pederzoli, T. Francavilla, A. Stabile report no disclosures. M. Maschio has received compensation from EISAI and Ethypharm for participation in conferences. S. Gasparini has received speaker honoraria from Eisai and Biogen. Filippo Dainese has received speaker or consultancy fees from Angelini Pharma, Eisai, and UCB. Pharma outside the submitted work. G. Strigaro received speaker and consulting fees from Eisai and Angelini.
Figures
References
-
- Abimbola S, Martiniuk AL, Hackett ML, Anderson CS. The influence of design and definition on the proportion of general epilepsy cohorts with remission and intractability. Neuroepidemiology. 2011;36(3):204–212. - PubMed
-
- Maguire M, Marson AG, Ramaratnam S. Epilepsy (partial). BMJ Clin Evid. 2010;2010:1214. - PubMed
-
- Gilliam F. Optimizing health outcomes in active epilepsy. Neurology. 2002;58(8 Suppl 5):S9–S20. - PubMed
-
- Beghi E, Niero M, Roncolato M. Validity and reliability of the Italian version of the quality‐of‐life in epilepsy inventory (QOLIE‐31). Seizure. 2005;14(7):452–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources