Mannose-Glycated Metal-Phenolic Microcapsules Orchestrate Phenotype Switch of Macrophages for Boosting Tumor Immunotherapy
- PMID: 40014038
- PMCID: PMC12021090
- DOI: 10.1002/advs.202415565
Mannose-Glycated Metal-Phenolic Microcapsules Orchestrate Phenotype Switch of Macrophages for Boosting Tumor Immunotherapy
Abstract
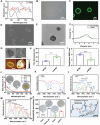
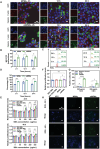
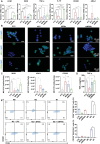
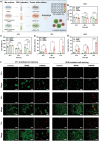
Microcapsules are advancing in immunotherapy, with both their core and shell being capable of loading immunoregulatory substances. Notably, microcapsules with intrinsic bioactivities can more directly modulate the immune microenvironment, while current research in this area remains scarce. Herein, immunomodulatory metal-phenolic microcapsules (mMPMs) is developed through the one-step assembly of dopamine-modified hyaluronic acid (HADA) and FeIII onto mannose-glycated bovine serum albumin microbubbles (Man-BSA MBs). Specifically, Man-BSA formed during the early stages of the Maillard reaction is sonicated to produce microbubbles as templates for capsule preparation. Subsequently, HADA is rapidly coated on the templates and coordinates with FeIII to form microcapsules after air escapes from MBs. Mass spectrometry analysis identifies abundant lysine glycation sites on Man-BSA, with the highest glycation site percentage reaching 94.88%. Man-BSA within mMPMs effectively promotes macrophage internalization, induces the accumulation of pro-inflammatory mediators, and thereby results in the M1 polarization of macrophages, as further corroborated by proteomic analysis. Consequently, the compelling anti-tumor effects of mMPMs are demonstrated both in vitro and in vivo. Overall, this work presents an immunomodulatory microcapsule that activates pro-inflammatory phenotype macrophages, which is a promising microcarrier to improve immunotherapeutic effects.
Keywords: M1 macrophage polarization; macrophage‐related immunotherapy; mannose‐glycated bovine serum albumin; metal‐phenolic microcapsules.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Li L., Puhl S., Meinel L., Germershaus O., Biomaterials 2014, 35, 7929. - PubMed
-
- Huang P., Wang X., Liang X., Yang J., Zhang C., Kong D., Wang W., Acta Biomater. 2019, 85, 1. - PubMed
-
- De Temmerman M., Rejman J., Lucas B., Vandenbroucke R. E., Libert C., Demeester J., De Smedt S. C., Adv. Funct. Mater. 2012, 22, 4236.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
