Patients with obstructive sleep apnea in Germany
- PMID: 40014191
- PMCID: PMC11868153
- DOI: 10.1007/s11325-025-03275-6
Patients with obstructive sleep apnea in Germany
Erratum in
-
Correction to: SURWEY real-world study of solriamfetol: initiation, titration, safety, efficacy, and follow-up experience for patients with obstructive sleep apnea in Germany.Sleep Breath. 2025 Apr 9;29(2):153. doi: 10.1007/s11325-025-03321-3. Sleep Breath. 2025. PMID: 40202544 Free PMC article. No abstract available.
Abstract
Purpose: Solriamfetol is approved for use in the European Union to treat excessive daytime sleepiness (EDS) associated with obstructive sleep apnea (OSA). SURWEY characterized real-world evidence regarding physician initiation and titration strategies and patient experiences with solriamfetol. We report SURWEY data for patients with OSA and EDS in Germany (N = 83).
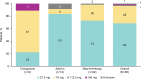
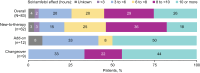
Methods: SURWEY was a retrospective chart review conducted among physicians in Germany. Eligible patients were age ≥ 18 years who reached a stable solriamfetol dose and completed ≥ 6 weeks of treatment. Patients were grouped by solriamfetol initiation strategy: changeover, add-on, new-to-therapy.
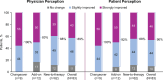
Results: Patients' mean (SD) age was 49 (14) years. New-to-therapy was the most common initiation strategy. Solriamfetol was initiated at 37.5 mg/day in most patients (n = 57, 69%) and titrated in 53 patients (64%); 30 (57%) completed titration within 2 weeks. In a post-hoc analysis, mean (SD) Epworth Sleepiness Scale (ESS) score was 16.0 (3.2) at baseline and decreased by 5.4 (3.6) at final follow-up (~ 16 weeks; p <.001). Improvement in patient- and physician-rated EDS was reported by ~ 90% of patients. Most patients (55%) reported effects of solriamfetol lasting ≥ 8 h; 91% of patients reported no change in nighttime sleep quality. The most frequent adverse events were headache (8%), decreased appetite (7%), and insomnia (6%).
Conclusion: Most patients in this study were new to therapy. Solriamfetol was typically initiated at 37.5 mg/day; titration was common. ESS scores improved with solriamfetol treatment, and most patients self-reported improvement in EDS symptoms. Common adverse events were consistent with those reported in previous clinical trials.
Keywords: Europe; Excessive daytime sleepiness; Germany; Obstructive sleep apnea; Real-world evidence; Solriamfetol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was conducted in accordance with applicable national and local requirements for good research study practices. Required country-specific documentation was reviewed and approved, per local regulations, before any patient chart data were included in the study. Because all patient chart data were de-identified and anonymous to the sponsor, informed consent was not required from patients, based on international regulations, including the General Data Protection Regulation. Conflict of interest: Y Winter has received honoraria for educational presentations and consultations from Angelini Pharma, Axsome Therapeutics, Avextra Pharma, Bayer AG, BIAL, Bioprojet, Bristol Myers Squibb, Eisai, Idorsia Pharmaceuticals, Jazz Pharmaceuticals, LivaNova, Novartis, and UCB Pharma. G Mayer has received honoraria for consultation and educational presentations by Idorsia, Pharmanovia, and Takeda. H Benes and L Burghaus have nothing to disclose. S Floam is an employee of Axsome Therapeutics. G Parks is a former employee of Axsome Therapeutics. U Kallweit is on the advisory board at Bioprojet Pharma, Harmony Biosciences, Jazz Pharmaceuticals, and Takeda Pharma. He is also a consultant to AOP Orphan Pharmaceuticals, Bioprojet Pharma, Harmony Biosciences, Jazz Pharmaceuticals, and Takeda Pharma and has accepted institutional grants/research support from Bioprojet Pharma, Harmony Biosciences, and Jazz Pharmaceuticals.
Figures
References
-
- Zhou J, Camacho M, Tang X, Kushida CA (2016) A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med 23:99–108. 10.1016/j.sleep.2016.02.008 - PubMed
-
- Kallweit U, Pevernagie D, Lammers GJ (2022) Sleepiness in obstructive sleep apnea: getting into deep water. Sleep Med 92:64–66. 10.1016/j.sleep.2022.02.015 - PubMed
-
- Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, Grillet Y, Levy P, Pepin JL, Pneumology, -FFP SCotSRotFFo (2013) Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res 22:389–397. 10.1111/jsr.12039 - PubMed
-
- Pepin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Levy P, Dervaux B, Lenne X, Mallart A (2009) Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J 33:1062–1067. 10.1183/09031936.00016808 - PubMed
-
- Bonsignore MR, Pepin JL, Cibella F, Barbera CD, Marrone O, Verbraecken J, Saaresranta T, Basoglu OK, Trakada G, Bouloukaki I, McNicholas WT, Bailly S, Pataka A, Kvamme JA, Hein H, Mihaicuta S, Grote L, Fanfulla F (2021) Excessive daytime sleepiness in obstructive sleep apnea patients treated with continuous positive airway pressure: data from the European Sleep Apnea Database. Front Neurol 12:690008. 10.3389/fneur.2021.690008 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources