Role of Glutamate Excitotoxicity in Glioblastoma Growth and Its Implications in Treatment
- PMID: 40014265
- PMCID: PMC11994879
- DOI: 10.1002/cbin.70005
Role of Glutamate Excitotoxicity in Glioblastoma Growth and Its Implications in Treatment
Abstract
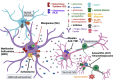
Glioblastoma is a highly malignant and invasive type of primary brain tumor that originates from astrocytes. Glutamate, a neurotransmitter in the brain plays a crucial role in excitotoxic cell death. Excessive glutamate triggers a pathological process known as glutamate excitotoxicity, leading to neuronal damage. This excitotoxicity contributes to neuronal death and tumor necrosis in glioblastoma, resulting in seizures and symptoms such as difficulty in concentrating, low energy, depression, and insomnia. Glioblastoma cells, derived from astrocytes, fail to maintain glutamate-glutamine homeostasis, releasing excess glutamate into the extracellular space. This glutamate activates ionotropic N-methyl-D-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on nearby neurons, causing hyperexcitability and triggering apoptosis through caspase activation. Additionally, glioblastoma cells possess calcium-permeable AMPA receptors, which are activated by glutamate in an autocrine manner. This activation increases intracellular calcium levels, triggering various signaling pathways. Alkylating agent temozolomide has been used to counteract glutamate excitotoxicity, but its efficacy in directly combating excitotoxicity is limited due to the development of resistance in glioblastoma cells. There is an unmet need for alternative biochemical agents that can have the greatest impact on reducing glutamate excitotoxicity in glioblastoma. In this review, we discuss the mechanism and various signaling pathways involved in glutamate excitotoxicity in glioblastoma cells. We also examine the roles of various receptor and transporter proteins, in glutamate excitotoxicity and highlight biochemical agents that can mitigate glutamate excitotoxicity in glioblastoma and serve as potential therapeutic agents.
Keywords: GLAST; cystine‐glutamate antiporter; glioblastoma; glutamate; mGlu3R; temozolomide.
© 2025 The Author(s). Cell Biology International published by John Wiley & Sons Ltd on behalf of International Federation of Cell Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

