Two-Year Follow-Up of Patients With Atrial Fibrillation Receiving Edoxaban in Routine Clinical Practice: Results From the Global ETNA-AF Program
- PMID: 40014354
- PMCID: PMC11885795
- DOI: 10.1002/clc.70091
Two-Year Follow-Up of Patients With Atrial Fibrillation Receiving Edoxaban in Routine Clinical Practice: Results From the Global ETNA-AF Program
Abstract
Background: Randomized clinical trials demonstrated similar efficacy and improved safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation (AF). Long-term data in routine clinical practice are needed.
Hypothesis: Patients with AF receiving edoxaban at baseline continue to have low annualized effectiveness and safety event rates in the second year of follow-up, with regional variations observed.
Methods: The Global ETNA-AF program is a prospective, noninterventional study of patients with AF receiving edoxaban. Patient characteristics and annualized clinical event rates were assessed overall and by region across the 2-year follow-up. Annualized event rates of bleeding and thromboembolic events were assessed within the first year and conditionally in patients who were event-free up to 12 months in the second year.
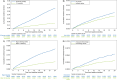
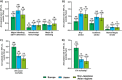
Results: This analysis comprised 26 805 patients from Europe (n = 13 164), Japan (n = 10 342), and non-Japanese Asian regions (n = 3299). Patients from Europe had the highest burden of comorbidities. The annualized event rates for major bleeding, any stroke, all-cause death, and cardiovascular death varied by region. The global annualized event rates in the first and second year were 1.31%/year and 0.86%/year for major bleeding, 1.06%/year and 0.65%/year for any stroke, 0.84%/year and 0.73%/year for cardiovascular death, and 3.05%/year and 3.18%/year for all-cause death.
Conclusion: Annualized event rates for any stroke and major bleeding remained low through 2-year follow-up for patients with AF receiving edoxaban at baseline. Differences in annualized event rates for all-cause and cardiovascular mortality between Europe, Japan, and non-Japanese Asian regions may reflect variations in baseline characteristics.
Trial registration: Europe, NCT02944019; Japan, UMIN000017011; Korea/Taiwan, NCT02951039; Hong Kong, NCT03247582; and Thailand, NCT03247569.
Keywords: anticoagulation; atrial fibrillation; direct oral anticoagulant (DOAC); edoxaban; major bleeding; oral anticoagulants; stroke prevention.
© 2025 The Author(s). Clinical Cardiology published by Wiley Periodicals, LLC.
Conflict of interest statement
R.D.C. declares fees, honoraria, and research funding from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, Menarini, Merck, Novartis, Portola, Roche, and Sanofi‐Aventis. M.U. and C.C. are employees of Daiichi Sankyo. E.‐K.C. declares research grants or speaking fees from Abbott, Bayer, Biosense Webster, BMS/Pfizer, Chong Kun Dang, Daewoong Pharmaceutical, Daiichi Sankyo, DeepQure, Dreamtech, Jeil Pharmaceutical, Medtronic, Samjinpharm, Samsung Electronics, Seers Technology, and Skylabs. Y.K. declares honoraria from Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, and Daiichi Sankyo. D.M. declares fees, honoraria, and research funding from Daiichi Sankyo. L.P. declares to have received funding for the statistics of this report from Daiichi Sankyo. P.B. declares to have received funding for the writing of this report from Daiichi Sankyo. C.C.W. declares honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Pfizer. T.Y. declares fees, honoraria, and research funding from Bayer, Bristol Myers Squibb, Daiichi Sankyo, Ono Pharmaceutical, and Toa Eiyo. P.K. declares research support for basic, translational, and clinical research projects from the British Heart Foundation, European Union, German Centre for Cardiovascular Research, and Medical Research Council (UK); and funding and honoraria from several drug and device companies active in atrial fibrillation in the past, but not in the last 3 years.
Figures


References
-
- Furie K. L., Goldstein L. B., Albers G. W., et al., “Oral Antithrombotic Agents for the Prevention of Stroke in Nonvalvular Atrial Fibrillation: A Science Advisory for Healthcare Professionals From the American Heart Association/American Stroke Association,” Stroke 43, no. 12 (2012): 3442–3453. - PubMed
-
- Giugliano R. P., Ruff C. T., Braunwald E., et al., “Edoxaban Versus Warfarin in Patients With Atrial Fibrillation,” New England Journal of Medicine 369, no. 22 (2013): 2093–2104. - PubMed
-
- Ruff C. T., Giugliano R. P., Braunwald E., et al., “Comparison of the Efficacy and Safety of New Oral Anticoagulants With Warfarin in Patients With Atrial Fibrillation: A Meta‐Analysis of Randomised Trials,” Lancet 383, no. 9921 (2014): 955–962. - PubMed
-
- Hindricks G., Potpara T., Dagres N., et al., “2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration With the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed With the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC,” European Heart Journal 42, no. 5 (2021): 373–498. - PubMed
-
- Otto C. M., Nishimura R. A., Bonow R. O., et al., “2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines,” Circulation 143, no. 5 (2021): e72–e227. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

